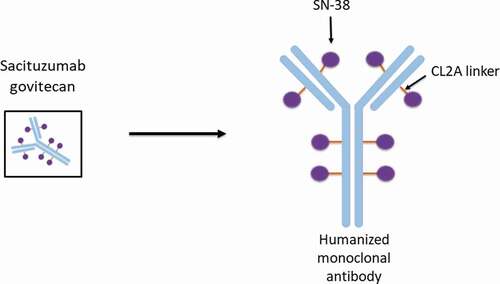
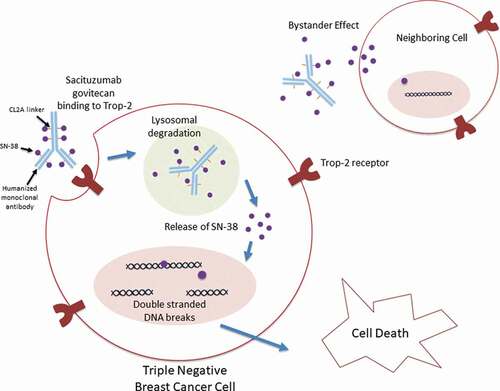
Figures & data
Table 1. Common adverse events in 258 patients with metastatic triple-negative breast cancer receiving saituzumab govitecan and 224 patients receiving single-agent chemotherapy (eribulin, vinorelbine, capecitabine, gemcitabine) in the Phase III clinical trial. [45]
Table 2. Phase III results of patients treated with sacituzumab govitecan and single-agent chemotherapy (eribulin, vinorelbine, capecitabine, gemcitabine) in metastatic triple-negative breast cancer. [45]