Figures & data
Table 1. Demographic features of all enrolled EGFR-mutant NSCLC patients progressed from first-/second generation TKIs and received third-generation TKIs based on genetic results via ddPCR and NGS.
Figure 1. The flowchart and the categorization of all advanced NSCLC patients with EGFR-sensitizing mutations included for analysis of the efficacy of subsequent third-generation TKIs based on different statuses of T790M mutation.
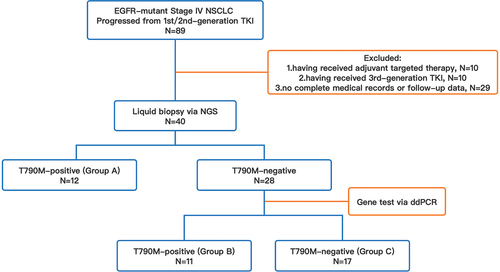
Figure 2. Survival analysis based on T790M status via liquid biopsy prior to subsequent third-generation EGFR-TKIs, which was categorized as follows: group A, T790M-positive via NGS (n=12); group B, T790M-negative via NGS, while tested positive via ddPCR (n=11); group C, T790M-negative via NGS/ddPCR (n=17). (a) survival analysis in patients with T790M-positive and -negative via NGS; (b) survival analysis in group A, B, and C.
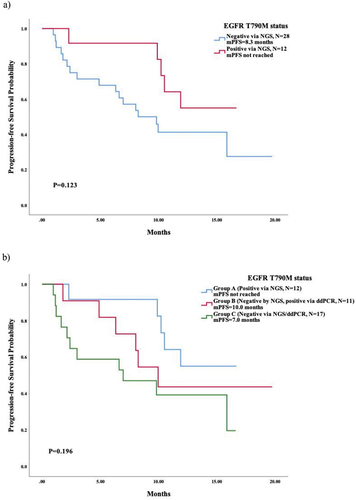
Figure 3. Survival analysis based on coexistance of EGFR sensitive mutation via NGS prior to subsequent third-generation EGFR-TKIs. (a) survival analysis in T790M-negative patients tested via NGS; (b) survival analysis in patients with T790M-negative tested via NGS while positive via ddPCR (group B).
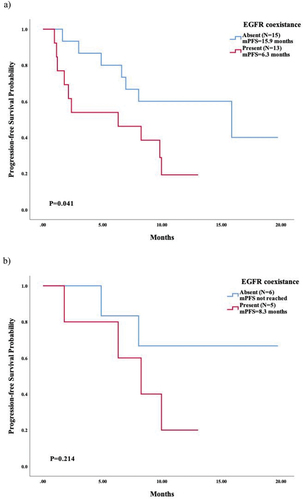
Figure 4. A typical example in an advanced NSCLC patient successfully treated with osimertinib 80mg QD, subsequent to first-line gefitinib treatment and second-line therapy. This patients had a negative EGFR or T790M mutation status prior to third-line osimertinib via NGS in peripheral blood, while tested T790M-positive (0.03%) in a further ddPCR test using ctDNA.
Table 2. The overall best response to third-generation TKIs in 32 included patients with at least one target lesion.
Table 3. The overall best response to third-generation TKIs in T790M-negative patients via NGS with at least one measurable lesion.
