Figures & data
Figure 1. Biomarkers may support patient management throughout the disease course, from primary ulcerative colitis prevention to minimization of adverse event risk. The optimal selection of accurate biomarkers is the foundation of personalized medicine.
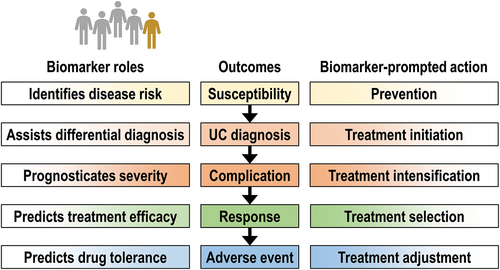
Table 1. The diversity of biomarker identification strategies in ulcerative colitis (UC) from the perspective of adoption and/or development in the near future.
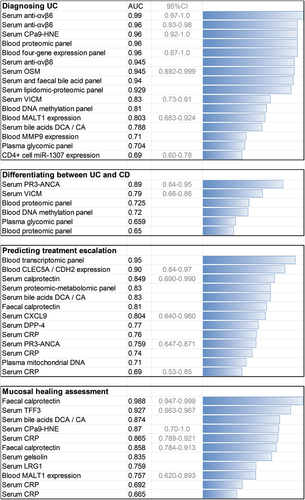
Figure 2. Comparison of the area under the receiver-operating characteristic curve (AUC) for some of the established and investigational ulcerative colitis biomarkers in various clinical contexts. The AUC values are presented to illustrate general trends in the field and not to compare individual studies, which strongly differ in design, e.g. a higher AUC is more easily achieved for short-term prognostication or when patients diagnosed with UC had more active disease. Only studies that reported an AUC are included, and in some cases, multiple studies provided data on the value of the same marker in the same application.