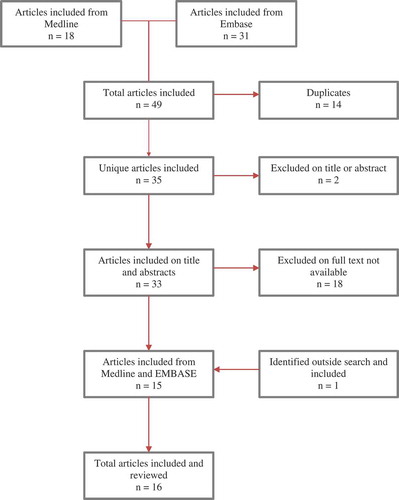
Figures & data
Table 1. Main characteristics and study design of the included studies.
Table 2. Characteristics of the vaccination programs and its vaccines.
Table 3. Influenza-related input parameters of the included studies.
Table 4. Effectiveness and cost-effectiveness of QIV as compared with TIV and key parameters towards cost-effectiveness results.
![Figure 2. The incremental vaccine price of quadrivalent influenza vaccine (QIV) as compared with trivalent influenza vaccine (TIV) used across included studies. Prices are converted to 2015 US$. * These studies assumed price parity between QIV and TIV. † For these studies incremental vaccine prices of inactivated vaccines were shown only. For live-attenuated influenza vaccines (LAIV), price parity was assumed between LAIV and quadrivalent LAIV. ‡ The study of Uhart et al. [Citation32] did not include vaccination costs, which reflects price parity.](/cms/asset/44e45df8-4f54-4fc7-9c31-4c0e8054638f/ierp_a_1343145_f0002_b.gif)
![Figure 3. Incremental cost-effectiveness ratios (ICER) in US$/quality-adjusted life year (QALY) gained of quadrivalent influenza vaccine as compared with trivalent influenza vaccine. ICERs are converted to 2015 US$. Static models are presented in black and dynamic models in grey. Results are presented from a payer’s perspective (Figure 3A) and the societal perspective (Figure 3B). CS: Cost-saving. *:The ICERs of Chit et al [Citation20] . and You et al. [Citation34] (highest) are not presented due to graphical issues. Chit et al. [Citation20] found an ICER of US $145,700 per QALY from the healthcare payer’s perspective and US $139,200/QALY from the societal perspective. You et al. [Citation34] (highest) found an ICER of US $254,200/QALY from the societal perspective. †UK1: Vaccine uptake rate of 52.5% in children aged 2-17 years. UK2: Vaccine uptake rate of 70% in children aged 2-17 years.](/cms/asset/032146da-4719-49bd-b468-16df4b5ac169/ierp_a_1343145_f0003_b.gif)