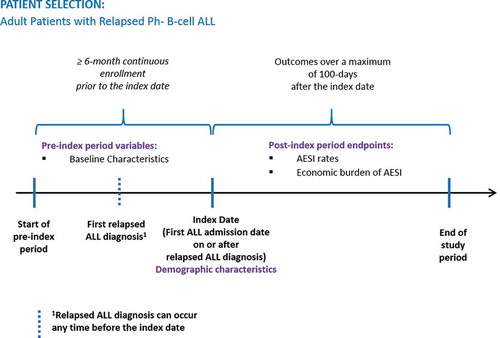
Figures & data
Figure 1. Patient selection flowchart for adults with relapsed Ph− B-cell ALL.
Ph−: philadelphia chromosome-negative; ALL: acute lymphoblastic leukemia; ICD: International Classification of Diseases; DRG: diagnosis related group; Ph+: Philadelphia chromosome-positive.
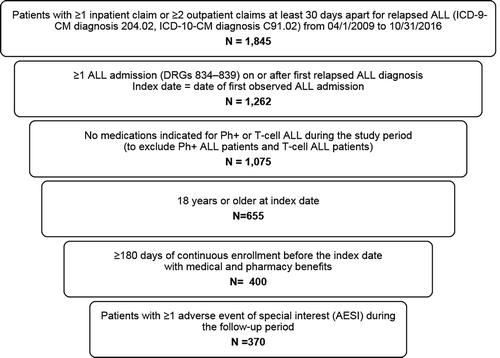
Table 1. Baseline characteristics of adult relapsed Ph− B-cell ALL patients with AESI.
Figure 3. AESI frequency by event type.
*AESI: adverse events of special interest; GI: gastrointestinal.
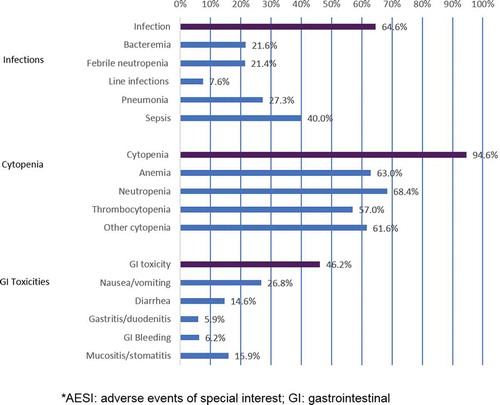
Table 2. Total, inpatient, and outpatient costs of AESI by event type during follow-up.
Table 3. Plan-paid inpatient and outpatient costs of AESI by event type during follow-up.