Figures & data
Table 1. Highly active relapsing-remitting adult incident patients’ characteristics (N = 9,596)
Table 2. Healthcare use (all disease and injuries combined) of highly active relapsing-remitting adult incident patients with at least two years of follow-up (N = 8,045)
Table 3. Cost of highly active relapsing-remitting adult incident patients with at least two years of follow-up and usable cost data (N = 7,956)
Figure 1. Mean annual cost per highly active relapsing-remitting adult incident patient with at least two years of follow-up and usable cost data (N = 7,956), in all patients and by inclusion criterion, by large category of items
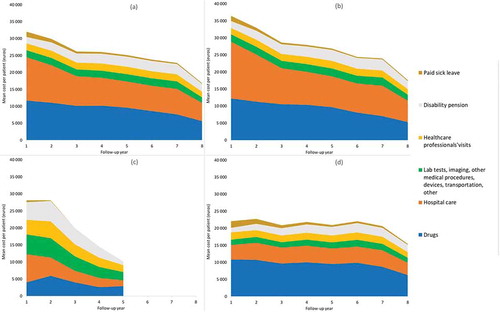
Figure 2. Mean annual cost per calendar year per highly active relapsing-remitting adult incident patient with at least two years of follow-up and usable cost data (N = 7,956), in all patients and by inclusion criterion, with 95% confidence intervals
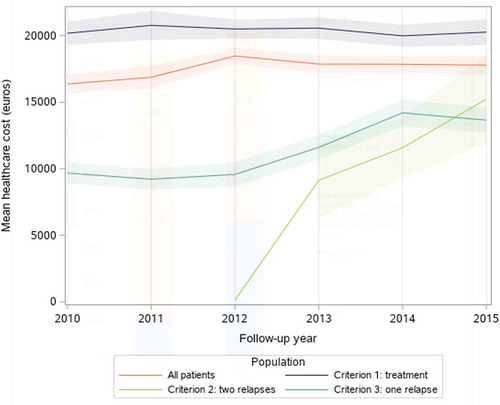
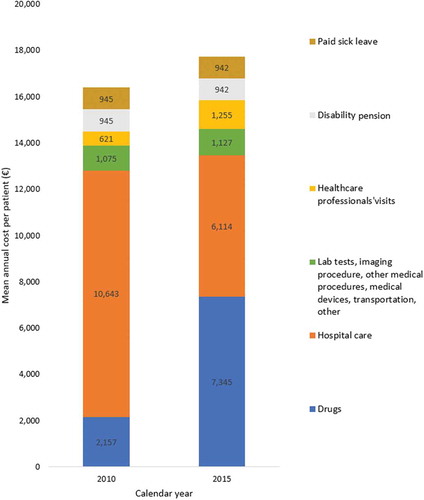
Figure 3. Mean annual cost per highly active relapsing-remitting adult incident patient with at least two years of follow-up and usable cost data, in 2010 (N = 1,097) and 2015 (N = 1,258)
Availability of data and material
Data from the National Health data system in France (Système National des Données de Santé, SNDS) are publicly available (https://www.snds.gouv.fr/SNDS/Processus-d-acces-aux-donnees) after acceptance from the French data protection authority (Comité National d’informatique et Liberté, CNIL). The datasets analyzed during the current study are available from co-author FR on reasonable request. All data generated or analyzed during this study are included in this published article.
