Figures & data
Figure 1. Study design. *Qualifying AF diagnosis; ≥1 inpatient or ≥2 outpatient claims with a primary or secondary diagnosis or paroxysmal (ICD-10 I48.0) or persistent (ICD-10 I48.1) AF occurring on different days during the identification period. Not to scale. Abbreviations: AF, atrial fibrillation.
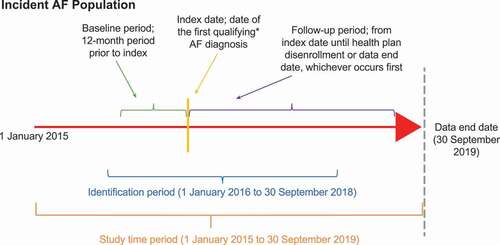
Figure 2. Patient attrition for the overall incident AF population Abbreviations: AAD, antiarrhythmic drugs; AF, atrial fibrillation.
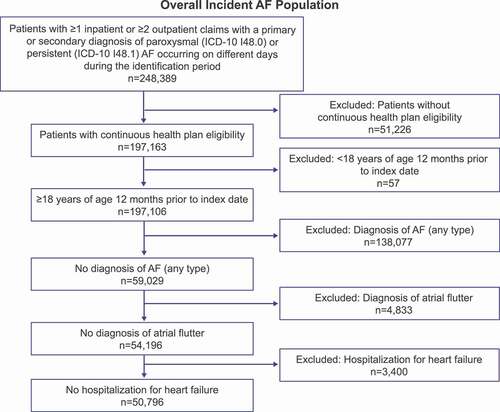
Table 1. Demographic and clinical characteristics
Table 2. HCRU event rates during the follow-up period
Table 3. HCRU event rates during the follow-up period for the overall population stratified by paroxysmal and persistent AF
Supplemental Material
Download MS Word (408.3 KB)Data Availability Statement
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized, and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com/.
