Figures & data
Table 1. MCDA framework for the evaluation of drugs in moderate-to-severe psoriasis.
Figure 1. Relative importance of each individual criterion in the assessment of drugs for the treatment of moderate-to-severe psoriasis (mean, min, max and median weights and standard deviations). The 100-points distribution method was applied, by which the experts assigned a weight to each criterion, provided its aggregation resulted in 100. CPG: clinical practice guidelines.

Table 2. Scores assigned per criterion, and main comments from the multidisciplinary experts committee or observation of subgroup results.
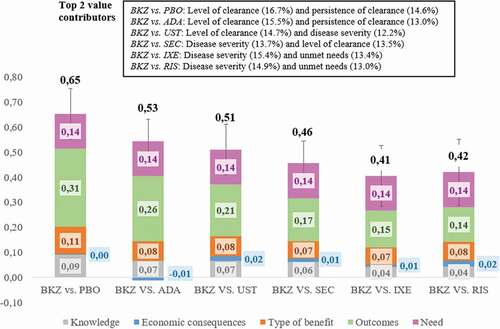
Figure 2. Value contribution of bimekizumab compared to placebo and five biological drugs according to the MCDA framework for the assessment of drugs in moderate-to-severe psoriasis. Mean value contribution per domain and overall value estimates are shown. Error bars show standard deviations across the twelve participants. BKZ: Bimekizumab. PBO: placebo. ADA: Adalimumab. UST: Ustekinumab. SEC: Secukinumab. IXE: Ixekizumab. RIS: Risankizumab.
Supplemental Material
Download Zip (1.9 MB)Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
