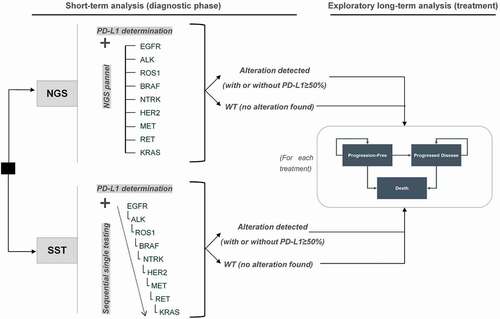
Figures & data
Table 1. Testing and positivity rates.
Table 2. Time spent and duration of pre-analytical and analytical phases.
Table 3. Unit cost for NGS and SST.
Figure 2. Distribution of alterations detected in hypothetical cohort.
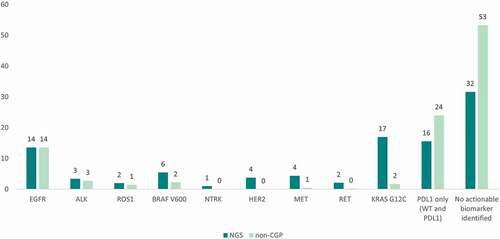
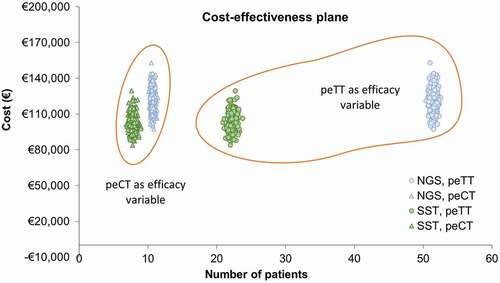
Table 4. Short-term cost and efficacy outcomes.
Figure 3. Time-to-results analysis.
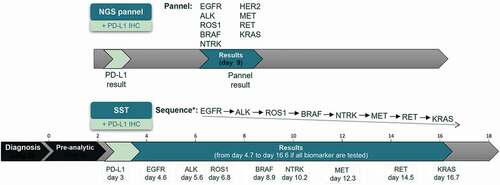
Table 5. Exploratory long-term results.
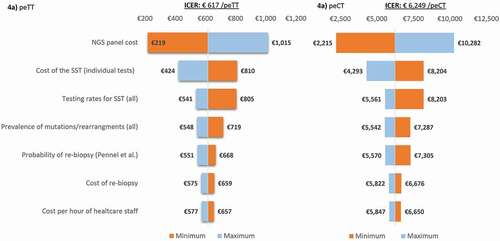
Figure 4. One-way sensitivity analysis, represented by tornado diagrams.