Figures & data
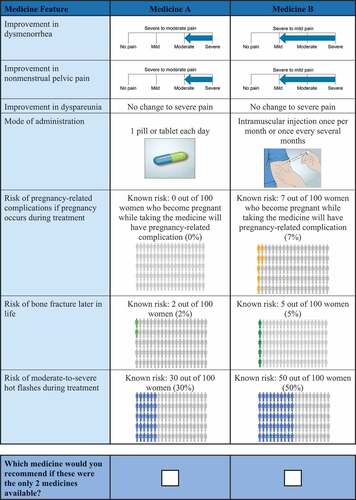
Table 1. Attributes and levels for the discrete-choice experiment.
Table 2. Demographic characteristics of physician respondents.
Figure 2. Relative preference weights and conditional relative attribute importance for respondents (N = 250).
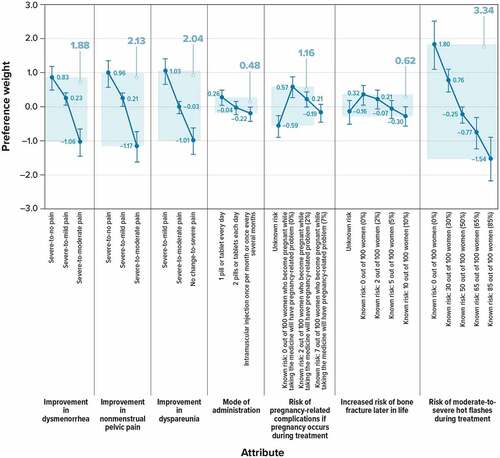
Table 3. Descriptions of the subgroups analyzed and results of tests for systematic differences in preferences (N = 250).
Table 4. Maximum acceptable risk calculations (N = 250).
Table 5. Treatment profiles and preference share results: baseline analysis results (N = 250).
Data availability statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.