Figures & data
Table 1. Baseline patient demographics, clinical characteristics, medications, and healthcare resource utilization after propensity score matching.
Figure 1. Patient disposition before propensity score matching (overall population and new-user subgroup).
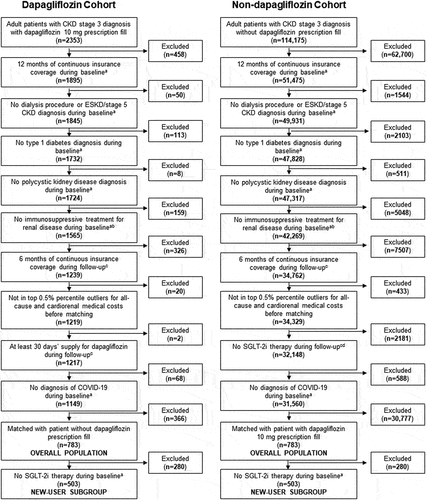
Figure 2. Cardiorenal medical costa to payers during 6 months’ follow-up for propensity score-matched patients with stage 3 CKD in the dapagliflozin and non-dapagliflozin cohorts in the (a) overall population and (b) new-user subgroup.
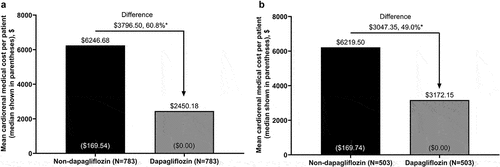
Figure 3. OOP cardiorenal medical costa to patients during 6 months’ follow-up for propensity score-matched patients with stage 3 CKD in the dapagliflozin and non-dapagliflozin cohorts in the (a) overall population and (b) new-user subgroup.
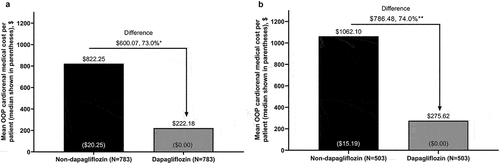
Figure 4. All-cause medical and pharmacy cost to payers during 6 months’ follow-up for propensity score-matched patients with stage 3 CKD in the (a) overall population and (b) new-user subgroup.
Supplemental Material
Download MS Word (51.3 KB)Data availability statement
This was a claims database analysis using IQVIA PharMetrics Plus closed claims data obtained under license from IQVIA Inc. The raw data cannot be publicly shared since it was obtained from IQVIA and as per signed agreement between AstraZeneca and IQVIA Inc. However, we have provided all relevant data in the manuscript that supports the research objectives and conclusions. We confirm that interested researchers can reach out to IQVIA Inc. to access the data. For further information on data access, please contact IQVIA.
