Figures & data
Table 1. Attributes and levels included in the DCE.
Figure 1. Survey respondents.
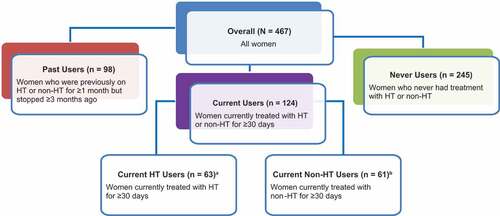
Table 2. Respondent Sociodemographic and Disease characteristics.
Table 3. Respondent vasomotor symptoms.
Table 4. Treatment characteristics among current treatment users.
Figure 2. Preference weights.
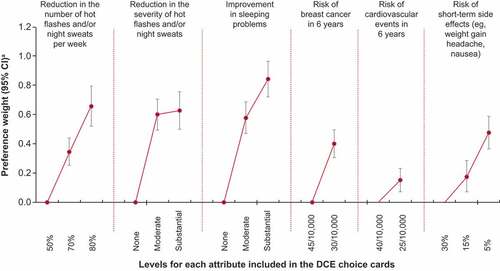
Table 5. Estimated preference weights and marginal willingness-to-pay.
