Figures & data
Figure 1. (a) Digital Measurement Solution (DMS) concept with building blocks combined into a comprehensive solution. (b) Detailed content on component modules.
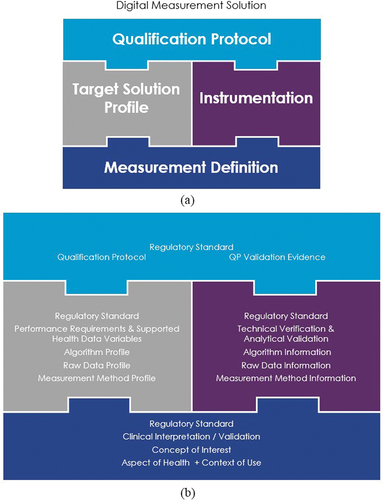
Figure 2. Illustrating how evidence can be repurposed for 2 Digital Measurement Solutions supported by different measurement methods (technologies) and using different algorithms in the same Context of Use, i.e. PAH. COI = Concept of Interest; DMS = Digital Measurement Solution; IMU = Inertial Measurement Unit; MAH = Meaningful Aspect of Health; PAH = Pulmonary Arterial Hypertension; QP = Qualification Protocol; SW = Software.
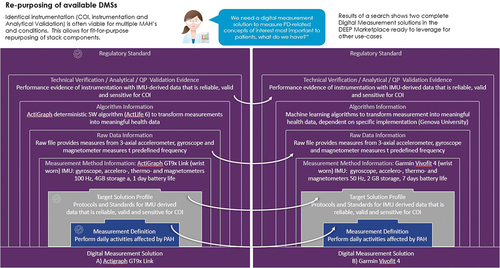
Figure 3. The measurement stack on the DEEP platform, with its components, enables the presentation of structured content and validation for digital measures. It has been built based on established industry standards. This figure maps the stack to one proposed dossier structure broadly accepted in industry and proposed in the literature (Walton et al. 2020 and its Figure 1). Section numbers in the figure refer to the dossier structure used to map to the proposed framework (slightly modified from figure 1 in Walton et al. 2020): 1. Executive Summary; 2. Intended Goal, 2.1 Endpoint Definition, 2.2 Endpoint Positioning, 2.3 Meaningful Aspect of Health intended as Clinical Benefit for Treatment, 2.4 Target Label Claim; 3. Concept of Interest for Measurement, 3.1 Concept of Interest for Measurement and Rationale, 3.2 Conceptual Framework; 4. Context of Use; 5. Content Validity Documentation; 6. Construct Validity and Ability to Detect Change, 6.1 Construct Validity, 6.2 Reliability; 6.3 Ability to Detect Change; 7. Clinical Interpretation; 8. Technology-Specific Plans Related to Use Affecting Clinical Trial Design and Data Analysis; 9. Description and Supporting Evidence of the Digital Health Technology, 9.1 Digital Health Technology (DHT), 9.2 Verification and Analytical Validity of the DHT, 9.3 Algorithm Description and Validation, 9.4 Usability Testing and Feasibility Research, 9.5 Safety and 9.6 Data Storage and Transfer Methodology. CSV = Computer System Validation, DMS = Digital Measurement Solution, GxP = Good Practices, QP = Qualification Protocol, TSP = Target Solution Profile.
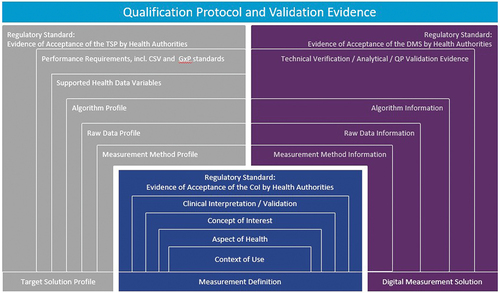
Figure 4. Illustrating how validation proof can be reused across sponsors, when using the same CoI for similar Context of Uses (in trials investigating medicinal products to treat COPD in our example) and across different DMSs based on similar measurement definitions (sponsor B is using the same DMS as presented in Figure 2).CoI = Concept of Interest; COPD = Chronic Obstructive Pulmonary Disease; CoU = Context of Use; CSV = Computer System Validation; GxP = Good Practice standards.