Figures & data
Figure 1. Pharmacologic agents approved or in development for Dravet syndrome
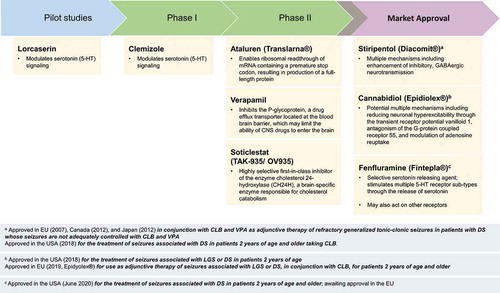
Table 1. Study and baseline characteristics of the pivotal phase III studies for the treatment of Dravet syndrome
Table 2. Efficacy in the pivotal phase III studies for the treatment of Dravet syndrome
Figure 2. Treatment-emergent adverse events occurring in ≥10% patients in the pivotal Dravet syndrome phase III trials
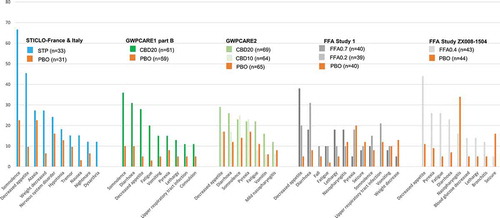
Figure 3. Drug interactions with stiripentol, cannabidiol and fenfluramine
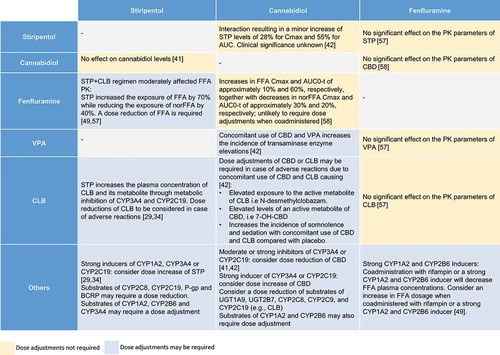
Table 3. Additional secondary efficacy outcomes in pivotal phase III studies
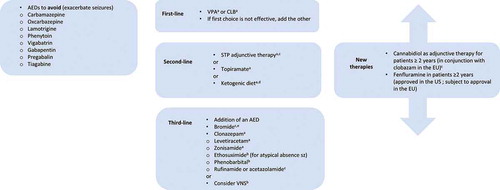
Figure 4. Current and potential future treatment pathway in Dravet syndrome