Figures & data
Table 1. Valbenazine studies in Tourette syndrome
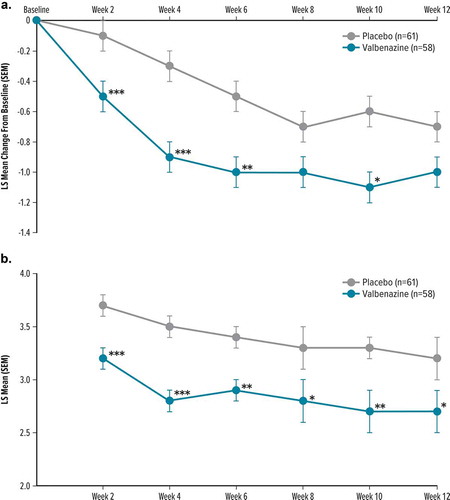
Figure 1. Change from baseline in YGTSS TTS by visit and treatment group in double-blind, placebo-controlled studies (primary outcome)
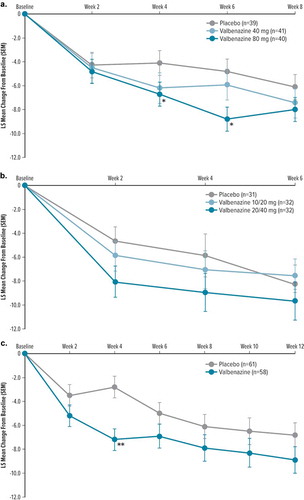
Table 2. Primary and select additional efficacy outcomes in double-blind, placebo-controlled studies
Table 3. Treatment-emergent adverse events in double-blind, placebo-controlled studies
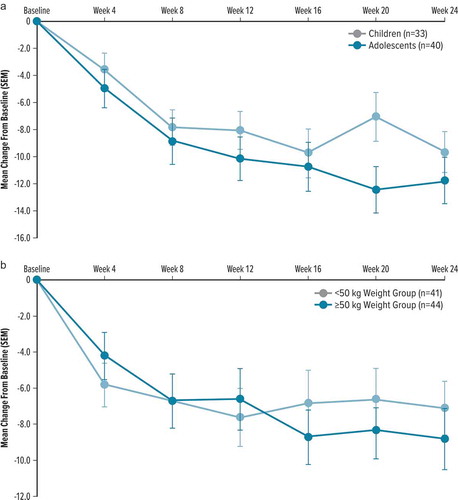
Figure 2. Secondary/exploratory efficacy outcomes by visit and treatment group in T-Force GOLD
Supplemental Material
Download MS Word (59.3 KB)Data availability statement
The datasets generated during and/or analyzed during the current study are not publicly available due to valbenazine being a product that has not been approved for the treatment of patients with Tourette syndrome but available from the corresponding author on reasonable request.