Figures & data
Figure 1. Flow Chart.
*Manual search of websites of national specialist relevant medical associations identified from https://wfneurology.org/about-us/member-societies.
**Manual search of websites from national (governmental) HTA bodies that determine reimbursement for medical treatments; limited to European countries where nabiximols (Sativex oromucosal spray) is known to be reimbursed. Relevant HTAs identified from https://www.eunethta.eu/about-eunethta/eunethtanetwork/.
^Two relevant documents from UK's HTA body, NICE, were included.
DM, disease modification; HTA, Health Technology Assessment; NICE, National Institute for Health and Care Excellence; UK, United Kingdom.
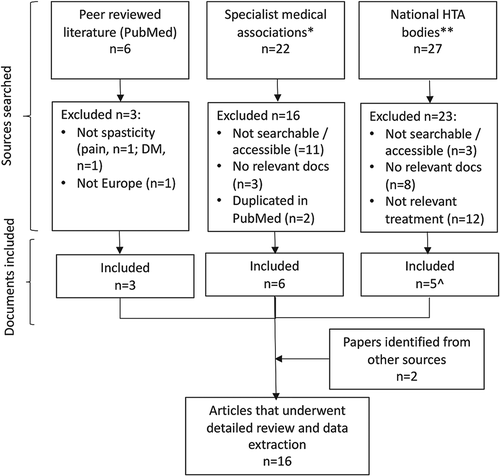
Figure 2. Composite treatment algorithm showing points of similarity and difference between the recommended treatment of generalized MSS in published national guidelines.
The information in this composite algorithm is based on all of the identified guidelines that described treatment pathways or lines of treatment (as shown in [Citation16,Citation19,Citation20,Citation25,Citation26,Citation27,Citation28,Citation29,Citation30,Citation31]), not only those that formally developed an algorithm. This algorithm is limited to generalized spasticity; elements from the published guidelines that related to focal spasticity have been excluded.
![Figure 2. Composite treatment algorithm showing points of similarity and difference between the recommended treatment of generalized MSS in published national guidelines.The information in this composite algorithm is based on all of the identified guidelines that described treatment pathways or lines of treatment (as shown in Table 1 [Citation16,Citation19,Citation20,Citation25,Citation26,Citation27,Citation28,Citation29,Citation30,Citation31]), not only those that formally developed an algorithm. This algorithm is limited to generalized spasticity; elements from the published guidelines that related to focal spasticity have been excluded.](/cms/asset/964d027c-e364-4cdb-827f-b482fbccbc81/iern_a_2075263_f0002_oc.jpg)
Table 1. Available national/regional guidelines describing clinical practice relating to treatment of MSS.
Table 2. Sativex-related recommendations in the context of Ms-associated spasticity, as provided in the national guidelines.
Table 3. Recommendations relating to the measurement and monitoring of clinical response to nabiximols.
Data availability statement
Findings in this review were all drawn from publicly available information, as referenced within the text of the publication. No additional data repository is necessary.
