Figures & data
Table 1. Baseline data about patient demographics and medical history (n = 129).
Table 2. Primary, secondary, and tertiary indications for cannabis-based medicinal products of study participants (n = 129).
Table 3. Median (IQR) baseline and follow-up scores for PHQ-9, GAD-7, SQS, EQ-5D-5L, and PGIC at 1, 3, and 6 months.
Figure 1. Box plots showing the change in PHQ-9 from baseline at 1, 3, and 6 months. Wilcoxon signed-rank tests were used for comparison between time intervals, *p < 0.050.
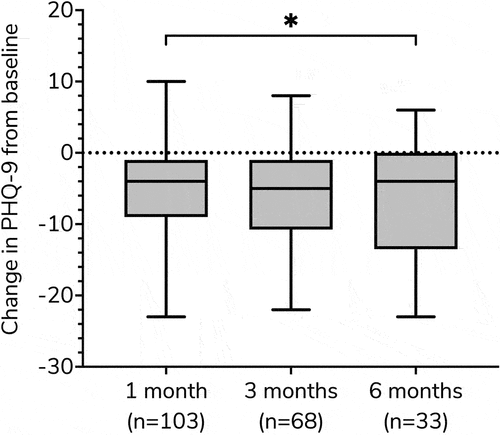
Table 4. Subgroup analysis of median (IQR) changes in PHQ-9 from baseline at 1, 3, and 6 months. Subgroups were compared using Mann-Whitney U tests.
Table 5. Adverse events recorded by participants (n = 129).