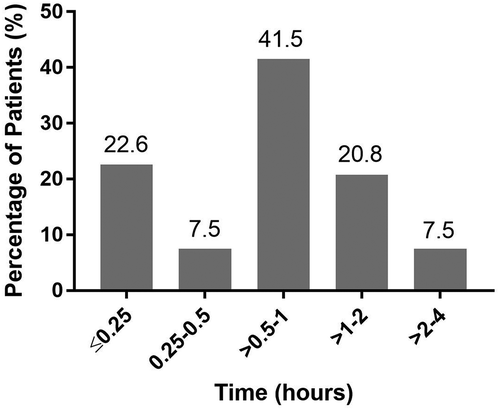
Figures & data
Figure 1. Disposition of patients in the (a) special drug use investigation of agalsidase beta for evaluation of long-term use and (b) drug use investigation of agalsidase beta as all-case surveillance
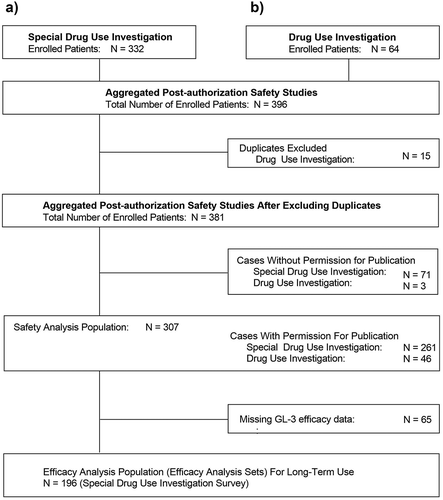
Table 1. Demographic and baseline clinical characteristics
Table 2. Occurrence of adverse drug reactions (with a PT Incidence ≥n = 3a) overall and by subgroup
Figure 2. Incidence of adverse drug reactions throughout the course of the study period A) Overall and by age and B) Phenotype subgroup
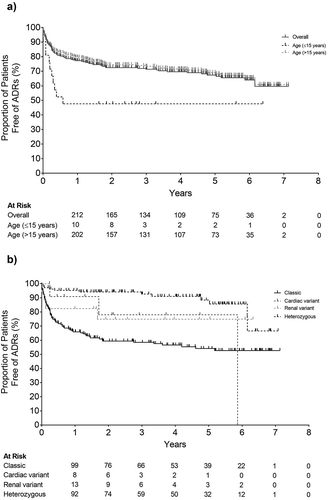
Table 3. Correlation between antibody production and adverse drug reactions and infusion-associated reactions
Table 4. Relationship between adverse events and IgG antibody titer
Figure 4. Change in blood GL-3 Level by (a) age subgroups, (b) disease phenotype, and (c) antibody status
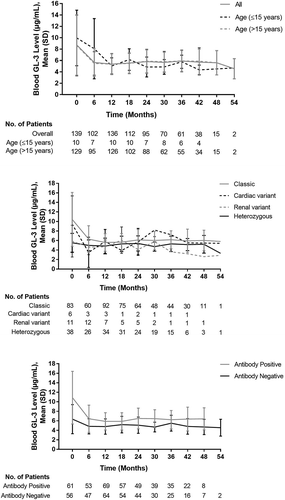
Table 5. Change from baseline in blood GL-3 level by patient background factors