Figures & data
Table 1. Characteristics of FDI, FCM, and FER [Citation5–10]
Figure 1. Chemical structures of the carbohydrate moieties in FDI, FCM, and FER, relative to dextran
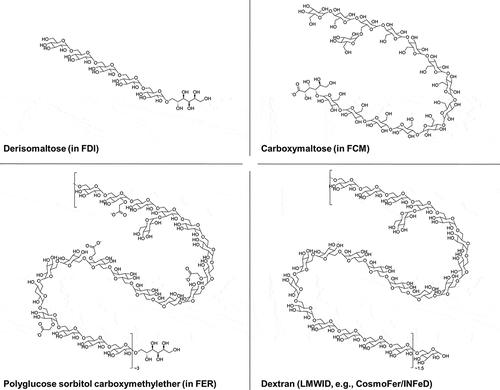
Figure 2. Bead-NTBI levels following a single dose of FDI, FCM, or iron sucrose [Citation27]
![Figure 2. Bead-NTBI levels following a single dose of FDI, FCM, or iron sucrose [Citation27]](/cms/asset/46e11d29-a1e0-4e6e-9b6e-4cb7758ec5c1/ieds_a_1912010_f0002_b.gif)
Figure 3. Incidence of hypophosphatemia reported during the PHOSPHARE-IDA trials [Citation28,Citation51]
![Figure 3. Incidence of hypophosphatemia reported during the PHOSPHARE-IDA trials [Citation28,Citation51]](/cms/asset/0ec68f25-6dfe-46fe-8604-bbd285063b30/ieds_a_1912010_f0003_b.gif)
Figure 4. Biochemical basis for hypophosphatemia and associated clinical consequences [Citation28,Citation48,Citation49,Citation57–67]
![Figure 4. Biochemical basis for hypophosphatemia and associated clinical consequences [Citation28,Citation48,Citation49,Citation57–67]](/cms/asset/ecd95321-3718-4063-b97f-6f1ec2fad170/ieds_a_1912010_f0004_b.gif)
Table 2. Ongoing clinical studies of IV iron in patients with HF and iron deficiency
