Figures & data
Figure 1. Dimensional graph about efficacy and acceptability in head-to-head studies.
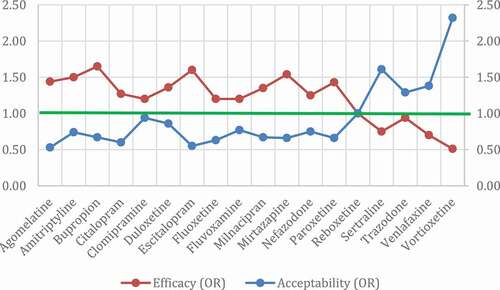
Table 1. Likelihood to be helped or harmed, and response vs. discontinuation because of an AE
Figure 2. Standardized effect size relative to placebo.
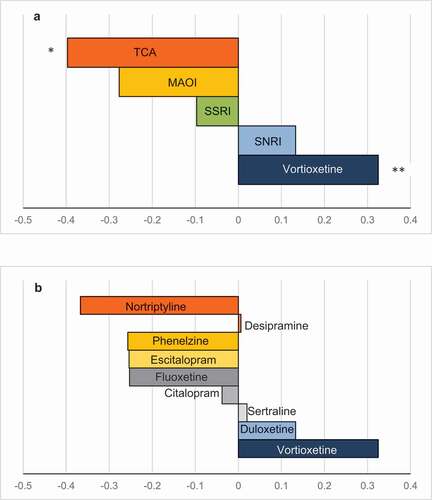
Figure 3. Change from baseline in SHAPS and MADRS anhedonia factor scores with vortioxetine.
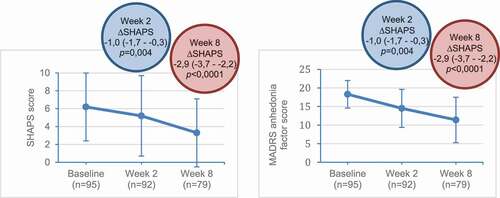
Figure 4. Change from baseline in HAM-D single items at week 6/8.
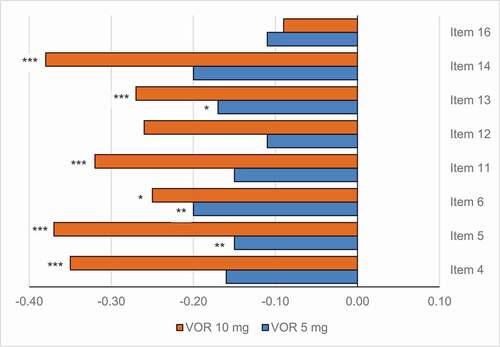
Figure 5. Risk difference for remission and for withdrawal due to AEs.
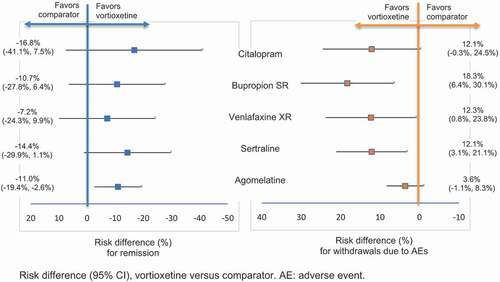
Table 2. Spontaneously reported AEs related to sexual dysfunction
Table 3. Analysis of treatment-emergent AEs
