Figures & data
Figure 1. Patient disposition. aPatients who used at least one dose of study drug. bPatients with at least one dose of study drug and any post-dose headache severity or symptom assessments. cPatients treated with at least one dose of study drug, any post-dose assessments, and treated for a qualifying migraine attack within 4 hours. LTN lasmiditan, (m)ITT (modified) intent-to-treat.
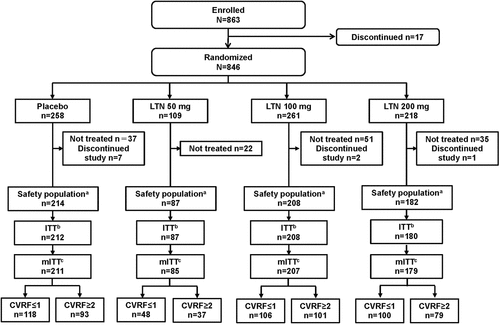
Table 1. Summary of cardiovascular risk factorsa
Table 2. Baseline demographics and clinical characteristicsa, stratified by CVRF subgroup
Table 3. Most common TEAEsa (≥5% in all-lasmiditan group) and vertigob
Table 4. Summary of likely CV TEAEsa
Figure 2. Relationship between CVRF subgroup categories and: (a) Proportion of patients who were headache pain-free at 2 hours post-dose (interaction p = 0.016); (b) Proportion of patients who experienced headache pain relief at 2 hours post-dose (interaction p = 0.235); (c) Proportion of patients who were MBS-free at 2 hours post-dose (interaction p = 0.601); (d) Proportion of patients who reported no disability at 2 hours post-dose (interaction p = 0.051). P-values are for the overall treatment-by-subgroup interaction term of the logistic regression model (model included treatment group, baseline usage of preventive migraine medications [Yes/No], subgroup [CVRFs {≤1, ≥2}], and treatment-by-subgroup interaction). CVRF cardiovascular risk factor, LTN lasmiditan, MBS most bothersome symptom.
![Figure 2. Relationship between CVRF subgroup categories and: (a) Proportion of patients who were headache pain-free at 2 hours post-dose (interaction p = 0.016); (b) Proportion of patients who experienced headache pain relief at 2 hours post-dose (interaction p = 0.235); (c) Proportion of patients who were MBS-free at 2 hours post-dose (interaction p = 0.601); (d) Proportion of patients who reported no disability at 2 hours post-dose (interaction p = 0.051). P-values are for the overall treatment-by-subgroup interaction term of the logistic regression model (model included treatment group, baseline usage of preventive migraine medications [Yes/No], subgroup [CVRFs {≤1, ≥2}], and treatment-by-subgroup interaction). CVRF cardiovascular risk factor, LTN lasmiditan, MBS most bothersome symptom.](/cms/asset/b31e9bf8-e9d2-4e5a-a84a-4588860cea7a/ieds_a_2078302_f0002_b.gif)
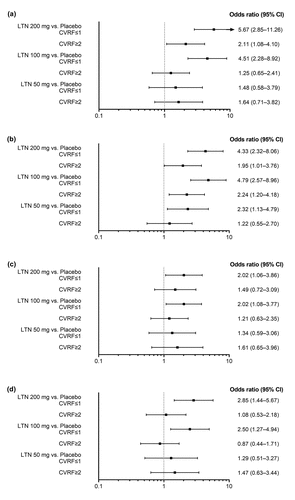
Figure 3. Comparisons between each lasmiditan dose and placebo in each of the CVRF subgroup categories (Forest plots) for the following efficacy outcomes: (a) Patients who were headache pain-free at 2 hours post-dose (arrow indicates upper CI extending beyond the x-axis limit); (b) Patients who experienced headache pain relief at 2 hours post-dose; (c) Patients who were MBS-free at 2 hours post-dose; (d) Patients who reported no disability at 2 hours post-dose. CI confidence interval, CVRF cardiovascular risk factor, LTN lasmiditan, MBS most bothersome symptom.
Supplementary_Lasmiditan_CVRFs.docx
Download MS Word (46.6 KB)Data availability statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
