Figures & data
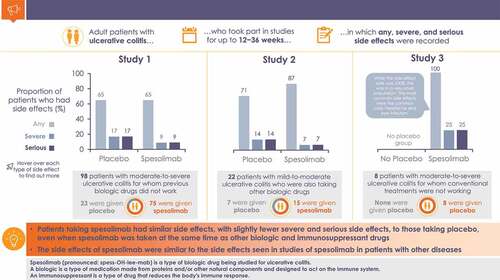
Table 1. Clinical trials of intravenous spesolimab as induction therapy in patients with ulcerative colitis.
Table 2. Baseline demographics and clinical characteristics in clinical trials of intravenous spesolimab in patients with ulcerative colitis.
Table 3. Concomitant and historical ulcerative colitis medications up to Week 12 in clinical trials of intravenous spesolimab in patients with ulcerative colitis.
Table 4. Overall summary of adverse events in clinical trials of intravenous spesolimab in patients with ulcerative colitis.
Table 5. Serious adverse events in clinical trials of intravenous spesolimab in patients with ulcerative colitis.
