Figures & data
Figure 1. Mechanism of action of perampanel.
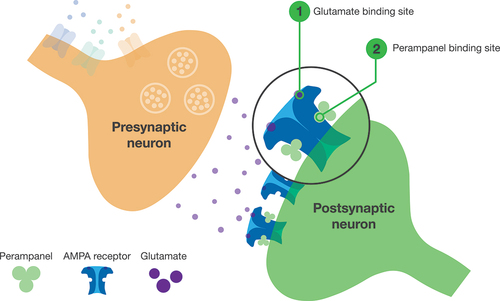
Table 1. Summary of study length, TEAEs, and most common TEAEs (occurring in ≥5% of patients) across studies that included patients treated with perampanel monotherapy or early adjunctive therapy (Safety Analysis Sets, where specified).
Table 2. Summary of the most common TEAEs (in ≥10% of patients for any ASM and at a higher frequency in the treatment group vs placebo) reported in clinical trials of adjunctive orala PER, BRV, CNB, ESL, LCM, LMT, and LEV in adultsb with FOS,c as described in the US prescribing information for each ASM.
Table
