Figures & data
Table 1. Medication incidents related to direct oral anticoagulants (DOACs) categorized as per the Reason’s accident causation model.
Figure 1. National Reporting and Learning Dataset used for the evaluation of incidents related to directly-acting oral anticoagulants.
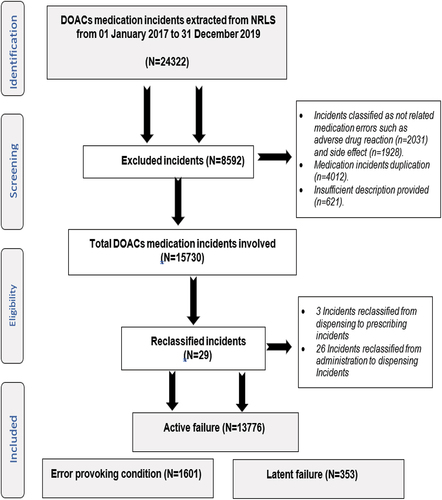
Table 2. Detailed descriptions of incidents leading to deaths with the assigned medication process stage, error type and nature of contributing factor.
Table 3. Examples of incidents associated with severe harm.
Table 4. Examples of incidents associated with moderate harm.
Table 5. Proportion and examples of safety incidents per stage of medication error category.
NRLS_Supplementary_EODS.docx
Download MS Word (361.1 KB)Data availability statement
All data corresponding to this work are provided with the manuscript.
