Figures & data
Table 1. Study design, demographics, baseline characteristics, and exposure for the six oncology clinical trials included in the analysis.
Table 2. Number of patients with any liver-related adverse event by preferred term, occurring in more than one patient per pooled treatment group.
Table 3. Liver biochemistry parameters and risk difference on treatment.
Figure 1. Mean liver biochemistry data change from baseline in (a) Alanine aminotransferase, (b) Aspartate aminotransferase, (c) Alkaline phosphatase, and (d) total bilirubin over time.
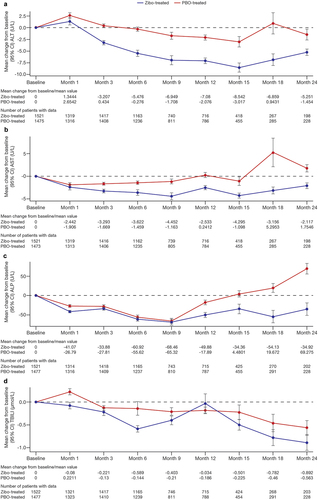
Table 4. Narratives of the seven zibotentan cases with alanine aminotransferase or aspartate aminotransferase >3× upper limit of normal and total bilirubin ≥2× upper limit of normal.
