Figures & data
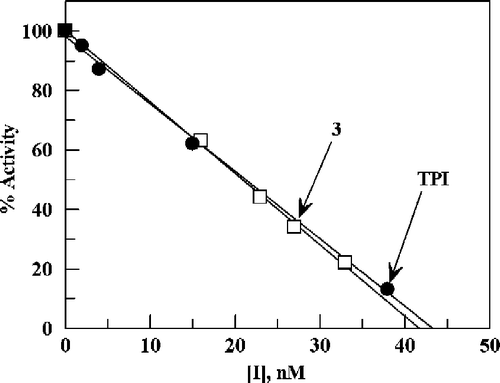
Figure 3 Plot of percent activity for E. coli TP versus concentration of TPI for various added enzyme volumes (indicated by each line in μL), using 20 μM thymidine as substrate in 0.1 M potassium phosphate (pH 7.4) at 25 °C. The straight lines are by linear regression analysis of data corresponding to the region 10–100% activity: the lines were used to determine apparent IC50 values. The inset is a plot of the apparent IC50 values versus the amount of E. coli TP added (the line is by linear regression analysis).
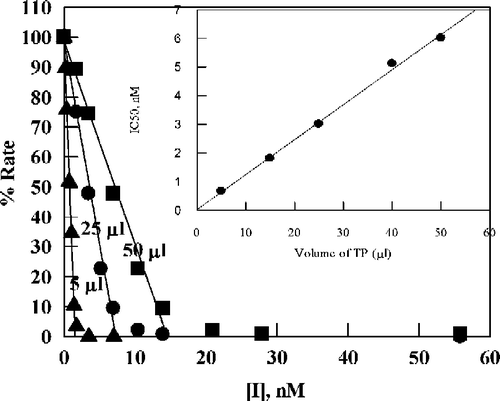
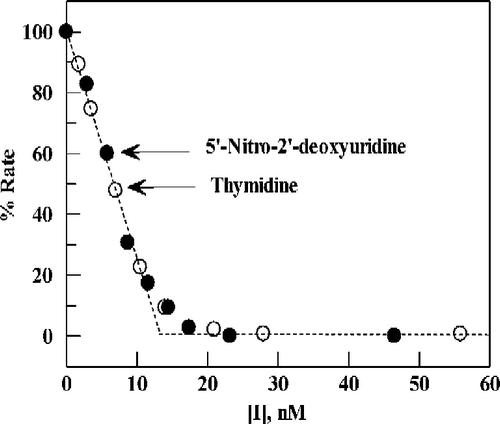
Figure 4 Plot of percent activity for E. coli TP versus the concentration of TPI, for substrates 5-nitro-2′-deoxyuridine (0.13 mM) (∘) or thymidine (20 μM)(•) in 0.1 M potassium phosphate (pH 7.4) at 25 °C. Points are experimental; the dashed line (angled, then horizontal) is the theoretical result expected for stoichiometric, instantaneous inhibition of enzyme present at 13 nM.