Figures & data
Figure 1 Fast-binding inhibition of FAS by different Extr concentrations, the overall reaction (♦), ketoacyl reduction reaction (▴), and enoyl reduction reaction (•) in the presence of Extr. The reaction system contains 0.1 mol/L potassium phosphate buffer, pH7.0; 1.0 mmol/L EDTA; 1.0 mmol/L DTT; 3 μmol/L acetyl-CoA; 10 μmol/L malonyl-CoA; 32 μmol/L NADPH and FAS 20 μg in a total volume of 2.0 ml, 37°C, by following the decease of NADPH at 340 nm within 1.5 min.
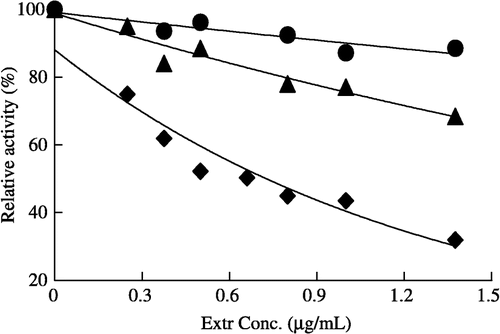
Figure 2 Comparison of the inhibitory effects of Extr and gallated catechins on FAS. The residual activity of FAS were measured after the enzyme was incubated with the inhibitors for 0.5 and 3.0 h, repectively. The concentrations of Extr, GCG, EGCG, and ECG in the incubation system are 1.3, 1.25,1.25, and 1.25 μg/mL, respectively. The FAS concentration was 1.9 μM. The experiment results are expressed as mean ± SD values.
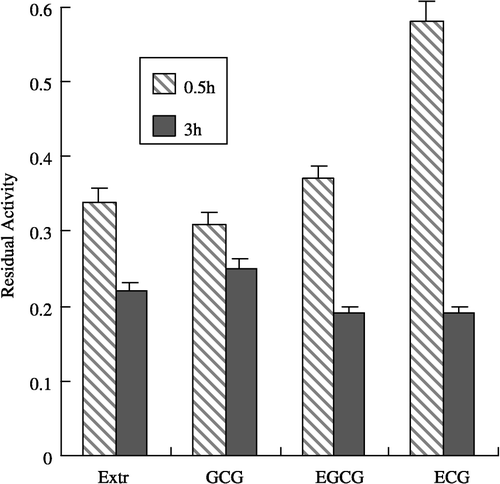
Figure 4 Kinetic time course of inhibition of the overall reaction (•) and ketoacyl reduction reaction (♦) in the presence of Extr. The insert is plot of ln R.A. (relative activity) versus time calculated from data. The FAS solution (0.6 μM) was mixed with Extr (0.5 μg/mL) and aliquots were taken to assay remaining activity at the indicated time intervals.
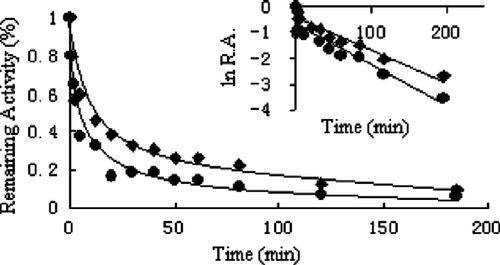
Figure 5 Effect on the apparent inactivation rate constant, kobs, with increasing Extr concentration. The concentration of FAS in the inactivation system was 2.1 μM (A) dependence of the observed first order inactivation rate constant (kobs) on Extr concentration (B) plot of the reciprocal of kobs versus the reciprocal of Extr, as an inhibitor, concentration.
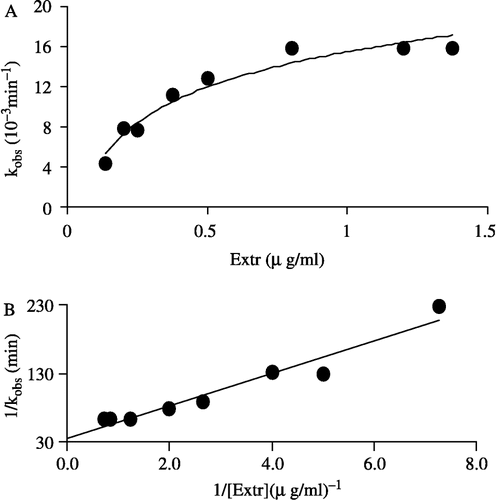
Figure 6 Lineweaver-Burk plot of the inhibition of overall reaction of FAS by Extr. The concentration of Extr in the reaction system was 0(0), 0.25 μg/mL (1), 0.5 μg/mL (2) and 1.0 μg/mL (3). The FAS concentration was 0.012 μM, and the fixed concentrations of Malonyl-CoA and Acetyl-CoA were 10 and 2.5 μM, respectively. The reaction rate was expressed by the consumed concentration of NADPH per min in the overall reaction.
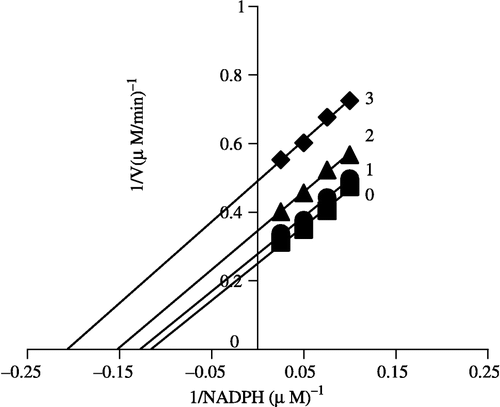
Figure 7 Lineweaver-Burk plot of the inhibition of overall reaction of FAS by Extr. The concentration of Extr in the reaction system was 0 (♦), 0.25 μg/mL (•), 0.5 μg/mL (▴), and 1.0 μg/mL (▪). The FAS concentration was 0.012 μM, and the fixed concentrations of NADPH and Acetyl-CoA were 32 and 2.5 μM, respectively. The reaction rate was expressed by the consumed concentration of NADPH per min in the overall reaction.
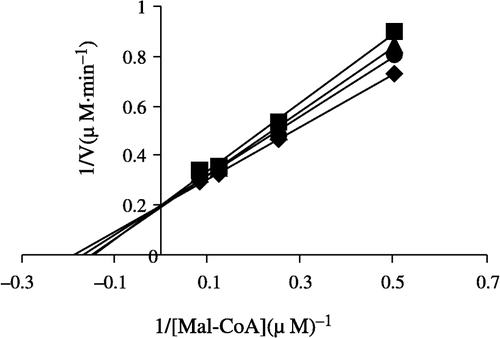
Figure 8 Lineweaver-Burk plot of the inhibition of the overall reaction of FAS by Extr. The concentration of Extr in the reaction system was 0(0), 0. 5 μg/mL (l), 1.0 μg/mL (2), and 1.25 μg/mL (3). The FAS concentration was 0.012 μM, and the fixed concentrations of NADPH and Malonyl-CoA were 32 and 10 μM, respectively. The reaction rate was expressed by the consumed concentration of NADPH per min in the overall reaction.
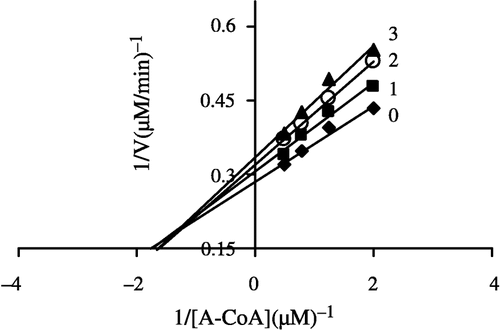
Table I. The inhibition type and inhibition constants for overall reaction of FAS with Extr and EGCG.
Figure 9 Lineweaver-Burk plot of the inhibition of β-ketoacyl reduction reaction of FAS by Extr. The fixed concentration of ethyl acetoacetate is 40 mM. The concentration of Extr in the reaction system was 0(0), 0. 25 μg/mL (1), 0.5 μg/mL (2), and 1.0 μg/mL (3). The FAS concentration was 0.012 μM, and the fixed concentrations of Acetyl-CoA and Malonyl-CoA were 2.5 and 10 μM, respectively. The reaction rate was expressed by the consumed concentration of NADPH per min in the ketoacyl reduction reaction.
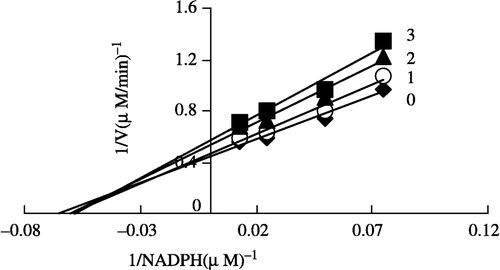
Table II. The extent of inhibition of various cancer cells by Extr.