Figures & data
Scheme 1 Synthesis of the 1,5-benzodiazepine derivatives. a) CH2Cl2, 1 h, rt, then 2 h, 8–12°C; b) EtOH, AcCl, 0°C, then 20 h, reflux; c) nitrobenzoic acid, DPPA, Et3N, DMF, 0°C, 5 h, then rt overnight; d) 37% HCl, 1 h, 40°C; e) RCOOH, DPPA, Et3N, DMF, 0°C, 5 h, then rt, overnight; or benzoyl chloride, Et3N, rt, overnight; or EDC, HOBt, Et3N, DMF, − 5°C and then rt overnight; f) H2O, NaOH, rt to 100°C, 2 h; g) cinnamic acid, DPPA, Et3N, DMF, 0°C, 5 h, then rt overnight; h) 1-methylpiperazine, DMSO, toluene, 12 h, 125°C.
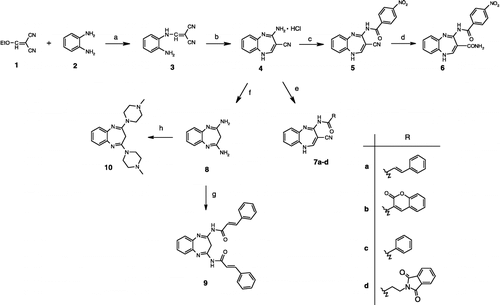
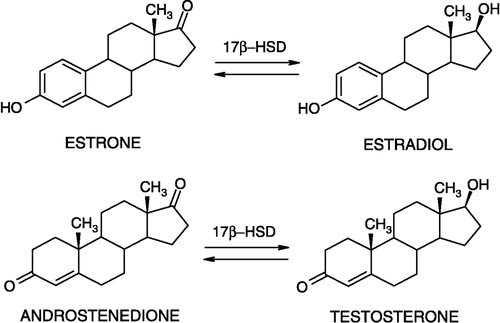
Table I. 1,5-Benzodiazepines as inhibitors of 17β-HSDcl.
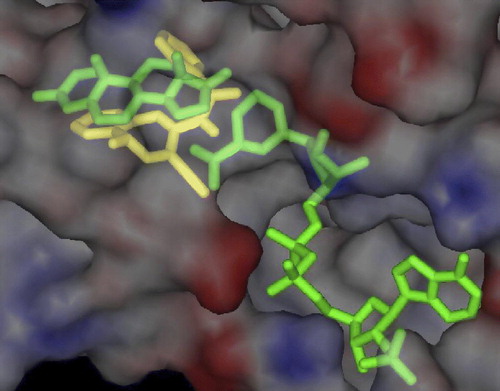
Figure 2 Superimposition of the computer modeling of compound 7c (in yellow) on the homology-built model of androstenedione and NADPH (both in green) bound to 17β-HSDcl. The highest ranked position of the inhibitor, as calculated by AutoDock 3.0, is presented. For clarity, only the surface of the protein is shown. (See colour online)