Figures & data
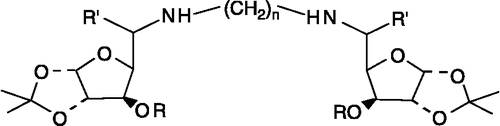
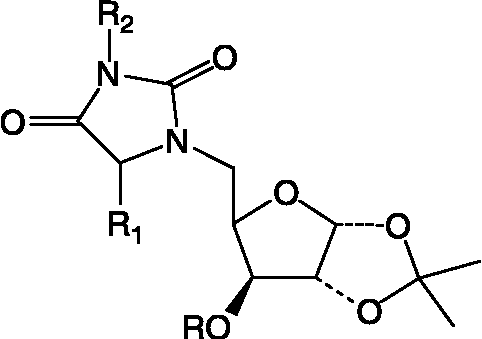
Table I. Structural details of N1, Nn- bis-Xylofuranosylated diaminoalkane (1).
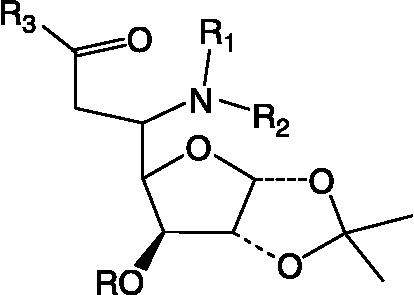
Table II. Structures of glycosyl amino acid (2), glycosyl hydroxamate (12), glycoconjugates (13, 14), glycosyl peptides (15L–18L), glycosyl thiourea (20), glycosyl urea (31L).
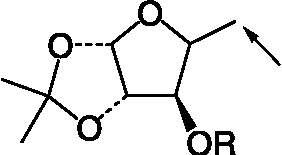
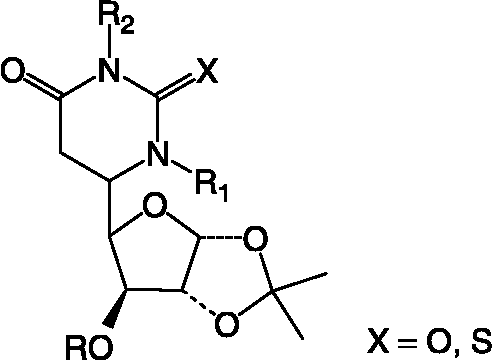
Table III. Structual details of glycosyl amino acid (3) (), glycosyl amino ester (4) (), glycosyl hydroxamic acids (5–11) (), N-protected glycopeptide (19) (), glycosyl ureas (21–30L) () and sugar (32) synthesized ().
Table IV. Structural detail of C-Nucleosides (35–57L) (see ) synthesized by a conventional method & on solid phase.
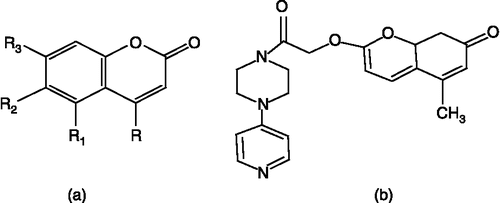
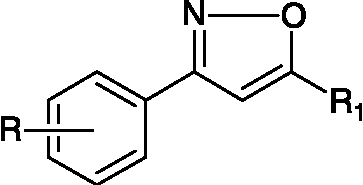
Table V. Structures of isoxazole derivatives (58–79).
Table VI. Structures of 2-hydroxymethylacrylic acid methyl ester (80), substituted hydroxymethyl acrylonitrile derivatives (81 and 82) and pyrrolidine derivative (83).
Table VII. Subcellular distribution of GST activity in P. yoelii.
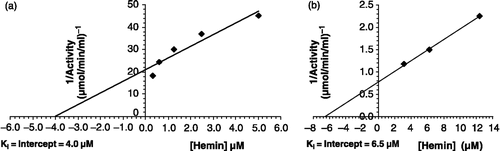
Figure 8 Linearity of P. yoelii cytosolic GST assay with respect to (a) time and (b) enzyme protein.
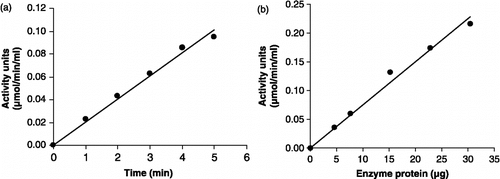
Figure 9 Linearity of P. falciparum recombinant GST assay with respect to (a) time and (b) enzyme protein.
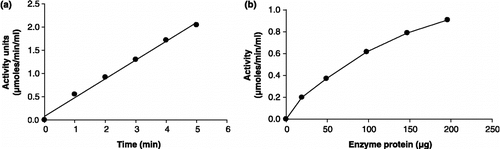
Figure 11 Type of inhibition of hemin on the activity of GST from (a) P. yoelii and (b) P. falciparum with respect to GSH.
Table VIII. Modulatory effect of synthetic compounds on GST from Plasmodium yoelii and Plasmodium falciparum.
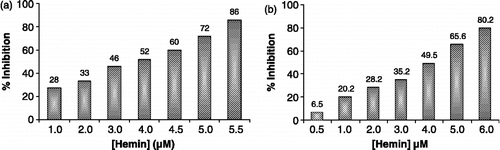
Table IX. IC50 values of standard GST inhibitor hemin and synthetic compounds with respect to recombinant GST from P. falciparum.