Figures & data
Figure 1 A schematic representation of the active site of HCV NS3 and its interaction with non-electrophilic and electrophilic inhibitors, assuming typical serine protease inhibition mechanisms. (A) mechanism-based inhibition by compounds with a C-terminal electron withdrawing group (EWG), and (B) product-based inhibitors with C-terminal carboxyl groups.
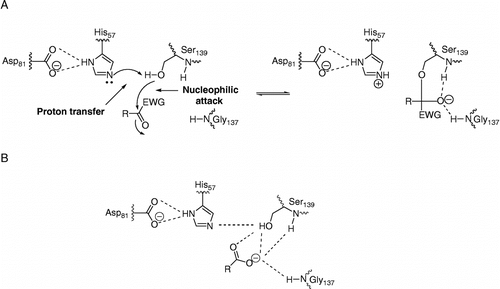
Table I. Inhibition of HCV NS3 by electrophilic and non-electrophilic inhibitors at different pH and ionic strengths† .
.
Figure 2 Dependence of the kinetic constants of HCV NS3 on pH. A) Vmax and B) Km as a function of pH, at low (•) and high (▪) ionic strength.
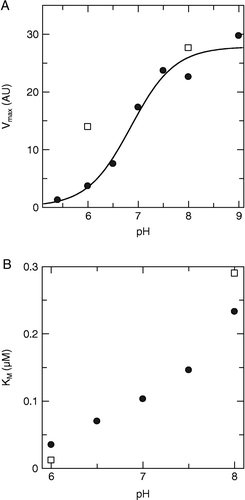
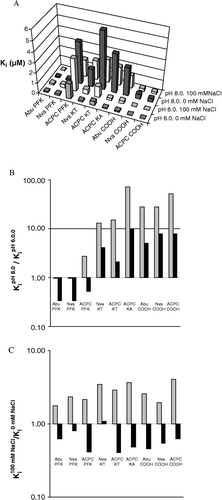
Figure 3 Effect of pH and NaCl concentrations on the inhibition constants for different inhibitors. A) Overview of Ki values for all compounds and conditions, see for details, B) Ratio of KipH 8.0/KipH 6.0 at 0 mM NaCl (grey) and 100 mM NaCl (black). C) Ratio of Ki100 mM NaCl/Ki0 mM NaCl at pH 6.0 (grey) and pH 8.0 (black).