Figures & data
Figure 1 Numbering of xanthones skeleton used in the HyperChem and NODANGLE calculations. (compound 1 in Table I).
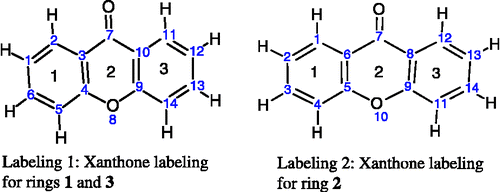
Figure 2 Numbering of the xanthones skeleton and angles used in the interpretations. Angles shown are for compound 1.
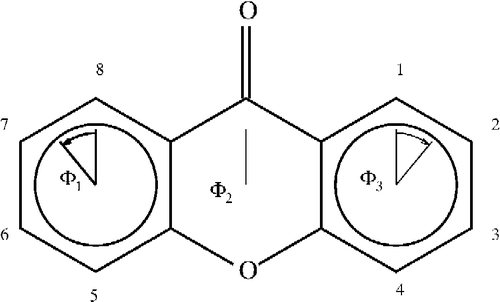
Table I. MAO Inhibitory activities of xanthone derivatives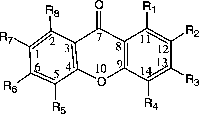 .
.
Table II. Orbital energies (eV) and angles (°) of the compounds in Table 1.
Table III. Calculated angles and their error terms.
Table IV. Descriptors used in this study.
Table V. Progress of FLIPSTEP for the reduced model.
Table VI. Flip status and flip significance for Equations (3) and (4).
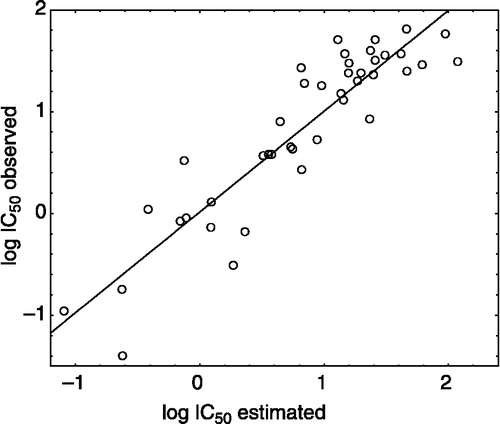
Table VII. Observed Log IC50 versus estimated.