Figures & data
Figure 1 Whole cell biocatalysts for steroid biotransformation as obtained by Autodisplay of bovine adrenodoxin (according to references [Citation3] and [Citation6]). In case that the P450 constituent consist of the enyzme CYP11B1, the whole cell biocatalysts efficiently converts the substrate 11-deoxycorticosterone (DOC) to the product corticosterone (B).
![Figure 1 Whole cell biocatalysts for steroid biotransformation as obtained by Autodisplay of bovine adrenodoxin (according to references [Citation3] and [Citation6]). In case that the P450 constituent consist of the enyzme CYP11B1, the whole cell biocatalysts efficiently converts the substrate 11-deoxycorticosterone (DOC) to the product corticosterone (B).](/cms/asset/5c007a27-16ec-42d5-a670-8b2dc0a59461/ienz_a_242415_f0001_b.gif)
Figure 2 HPTLC screening of steroid converting enzyme activity in strains of the E. coli strain collection and subsequent phosphoimager analysis using 3H-labeled DOC as a substrate. DOC = 11-deoxycorticosterone, B = corticosterone, P = product. lane 1: E. coli strain E132, 2: E133; 3: 134; 4: E135; 5: E144; 6: E151; 7: E153; 8: E152; 9: negative control without cells; 10: positive control containing all the constituents as described in Figure 1, products of the biotransformation of 3H-DOC with CYP11B1.
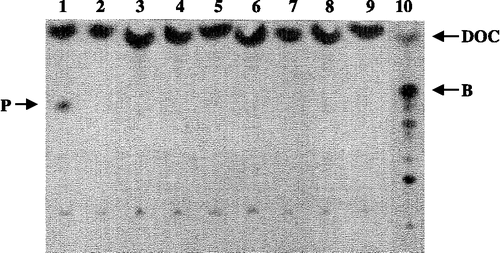
Figure 3 HPTLC and phosphoimager analysis of conversions using 3H-labeled DOC as a substrate in order to verify the biotransformation reaction of E. coli strain E132. The lanes contain the following samples: 1: control reaction of the mitochondrial CYP11B1 system, 2: E. coli E132 combined with the control reaction of 1), 3: components of 2) without adrenodoxin, 4: components of 2) without adrenodoxin reductase, 5: components of 2) without adrenodoxin and adrenodoxin reductase, 6: components of 2) without adrenodoxin and CYP11B1, 7: substrate conversion with E. coli E127 (negative control). The product of the biotransformation is marked on the right with a P and the products of the mitochondrial CYP11B1 system are designated on the left as B (corticosterone), aldo (aldosterone), 18(OH)B and 18(OH)DOC.
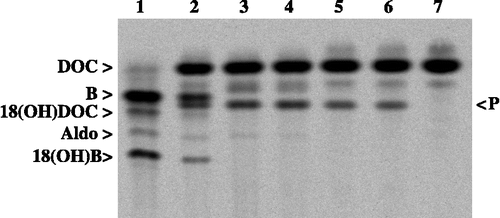
Figure 4 MALDI spectrometry analysis of the product 4-pregnen-20,21-diol-3-one (P) and the substrate DOC (inset) of the biocatalytic reaction as described. Signals at 143.11 and 284.04 correspond to the matrix 5-aminochinolin, whereas the signal at 315.06 represents an unknown impurity caused during the MS sample preparation.
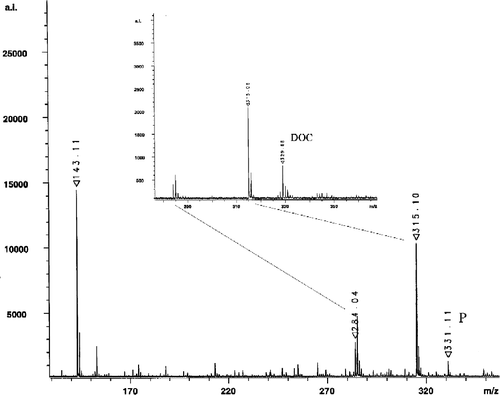
Table I. 13C NMR data in CDCl3 for substrate DOC and the product of biotransformation by E. coli E132, 4-Pregnen-20, 21-diol-3-one.
Scheme 1 (A): converting DOC to 20-dihydro-DOC (4-pregnen-20,21-diol-3-one), a whole cell biotransformation performed by E. coli E132. (B): Progesterone and the 20-oxime derivatives thereof as potent dual inhibitor of 5α-reductase types I and II and 17α-hydroxylase-C17,20-lyase (according to Ling et al. [Citation18]).
![Scheme 1 (A): converting DOC to 20-dihydro-DOC (4-pregnen-20,21-diol-3-one), a whole cell biotransformation performed by E. coli E132. (B): Progesterone and the 20-oxime derivatives thereof as potent dual inhibitor of 5α-reductase types I and II and 17α-hydroxylase-C17,20-lyase (according to Ling et al. [Citation18]).](/cms/asset/7261b176-b96f-4f52-9b09-9c3bbf6d7f75/ienz_a_242415_f0005_b.gif)
Table II. Inhibition of human and rat 5α-reductases by 4-pregnen-20,21-diol-3-one (20-dihydro-DOC).