Figures & data
Figure 1 a) Model of the secondary structure of FALDH highlighting the presence of the Rossmann fold; b) The hydrophobic pocket of FALDH generated from the molecular graphics package, YASARA Citation35-37. The substrate has been proposed to insert into the cleft to aid catalysis by association with the active site residue, Cys-241 (underlined, centre).
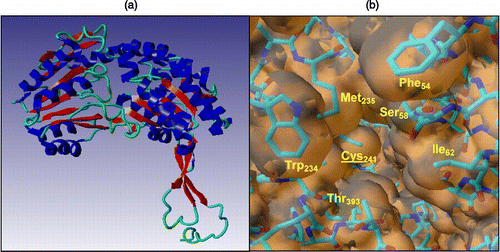
Table I. 1: FALDH (4 μg) activity with straight-chain substrates (50 μM), in the presence of N-lauroylsarcosine (C5-C12) or Triton X-100 (C14–C18).
Table II. 2: Kinetic parameters (Km, Vmax and kcat) for various straight- and branched-chain aldehyde substrates using 0.5 μg FALDH.
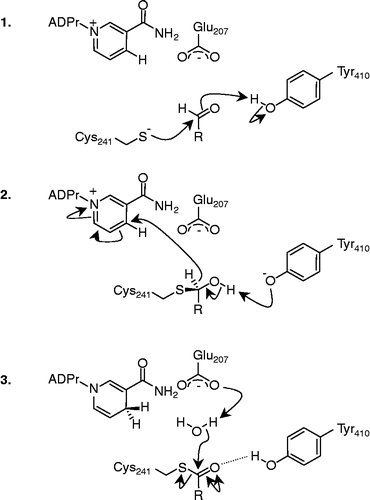
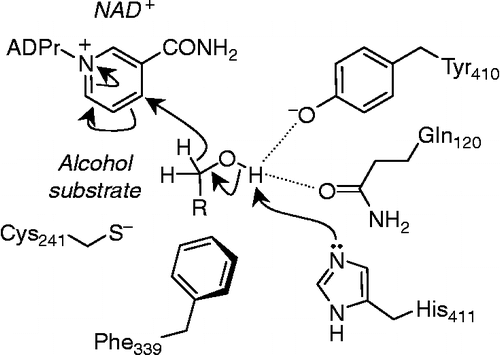
Figure 2 The three stages of the proposed mechanism for aldehyde oxidation: 1. attack of the Cys-241 thiolate anion on the aldehyde carbonyl. 2. Rearrangement of the thiohemiacetal intermediate and hydride transfer to NAD+. 3. Hydrolysis of the thioester to regenerate Cys241 and generate the respective acid. R = fatty acid side-chain. ADPr, Adenosine diphosphate ribose;