Figures & data
Figure 1 Chemical Structures of Representative α,γ-Diketo-Based Integrase and the Nucleoside Reverse Transcriptase Inhibitor (the 2′,3′-didehydro-2′,3′-dideoxythymidine).
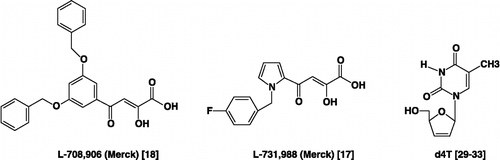
Figure 3 Design and proposed mechanism of conversion of double-drugs to d4T and IN inhibitor: Hydrolysis of the peptide linkage between the DKA and the amino acid residue (L-alanine).
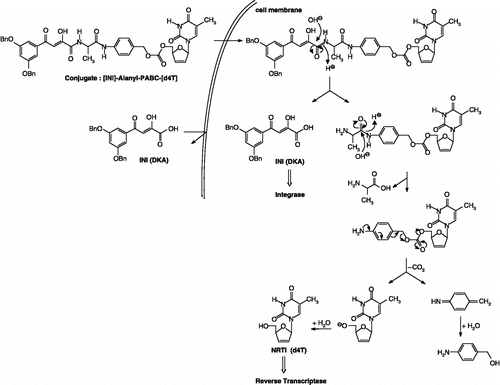
Figure 4 Design and proposed mechanism of conversion of double-drugs to d4T and IN inhibitor: Hydrolysis of the peptide linkage between the amino acid residue (L-alanine) and the PABC spacer.
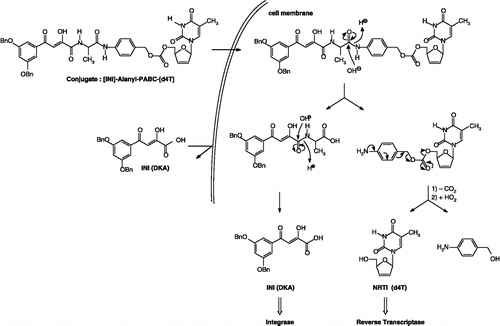
Table I. Antiviral and Cytotoxicity Evaluation of Precursors 2a–c, 3a–c, 4a–c and 5a–c and the conjugates [INI]-spacer-[d4T] (7a–c and 8a–c) against Selected HIV Strains.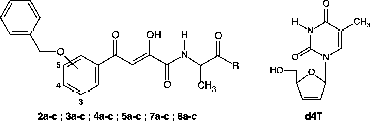
![Figure 2 Modifications carried out on the [L-708,906]-PABC-[d4T] heterodimer prototype.](/cms/asset/b8f26943-5ac3-4509-951f-20848c1176ab/ienz_a_242424_f0002_b.gif)
![Scheme 1 Synthesis of [DKA]-Alanyl-PABC-[d4T] (7a–c) and [DKA]-Alanyl-OABC-[d4T] (8a–c) conjugates.](/cms/asset/9b6d743a-c0d2-4f90-9ff0-ee8e292d7993/ienz_a_242424_f0005_b.gif)