Figures & data
Figure 1. Relative activity of pepsin in the presence of Al3 + ions. Proteolytic activity of pepsin expressed as percentage depends on Al3 + ions present. The degree of activation is expressed as % of increased activity considering the activity of pepsin in the absence of Al3 + ions as 100%. All results are expressed as a mean percentage of enzyme activity relative to the corresponding control value, from at least three independent experiments performed in triplicate.
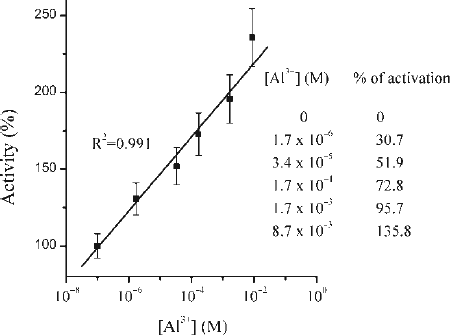
Figure 2. Lineweaver-Burk plot of a series of kinetics measurements in a presence of different Al3 + ions concentrations at pH2; (inset) Hyperbolic plots (sigmoid fit) representing initial pepsin velocity versus haemoglobin concentration in the absence and presence of different Al3 + ions concentrations at pH2.
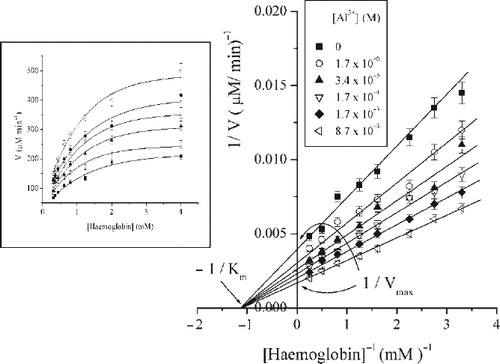
Table I. Influence of Al3 + on Km, Vmax, kcat and catalytic effectiveness of enzyme (kcat Km−1).
Figure 3. The plots of 1 / (change in slope or intercept), (1/Δ) against 1 / [Al3 + ], where Δ is defined as slope or intercept in the absence of activator (Al3 + ions) minus that in its presence.
![Figure 3. The plots of 1 / (change in slope or intercept), (1/Δ) against 1 / [Al3 + ], where Δ is defined as slope or intercept in the absence of activator (Al3 + ions) minus that in its presence.](/cms/asset/2bb8cbe5-243e-40b3-9f14-ef819fb9ad12/ienz_a_284243_f0003_b.gif)
Scheme 1 Reaction scheme for non-essential activation; Abbreviations are E—enzyme, S—substrate, A—activator, P—product.
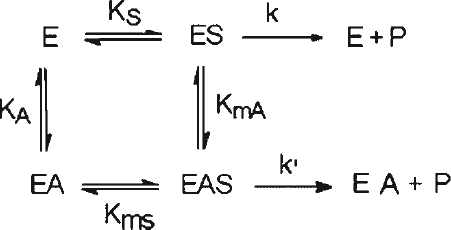
Figure 4. Activation of pepsin by Al3 + ions; The intercepts on the abscissa of the primary plot of [Hb]/V against reciprocal of activator concentrations provide the values of A50 used to obtain the plots of 1/A50 against v0/V shown in the inset.
![Figure 4. Activation of pepsin by Al3 + ions; The intercepts on the abscissa of the primary plot of [Hb]/V against reciprocal of activator concentrations provide the values of A50 used to obtain the plots of 1/A50 against v0/V shown in the inset.](/cms/asset/e13a7c50-ace2-45b8-9300-142c562db674/ienz_a_284243_f0005_b.gif)
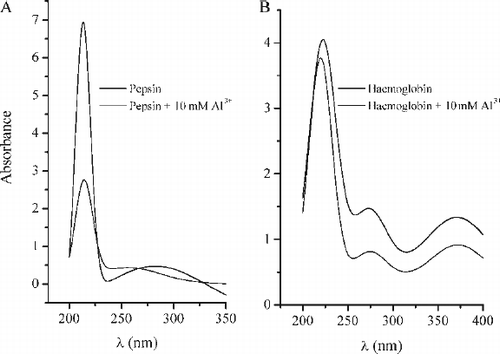
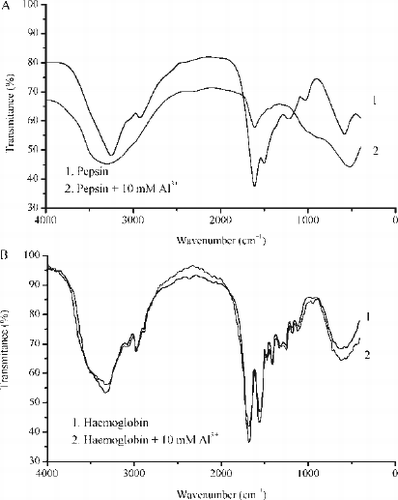
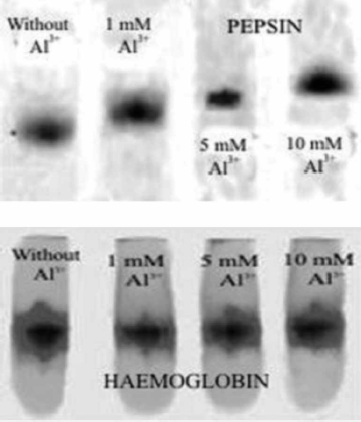
FIG. 5A –UV absorption spectra of pepsin (solid curve) and pepsin treated with 10 mM Al3 + (dotted curve). B - UV absorption spectra of haemoglobin (solid curve) and haemoglobin treated with 10 mM Al3 + (dotted curve). Other spectral curves obtained in a presence of 1 mM and 5 mM Al3 + are not presented because of clarity of the graphs.