Figures & data
Table I. Kinetic parameters for dUTP and dUDP hydrolysis by Campylobacter jejuni dUTPase.
Table II. Kinetic parameters for dUTP hydrolysis accomplished by monomeric, dimeric and trimeric dUTPases.
Figure 1. Complete hydrolysis of dUTP by dUTPase under multiple-turnover conditions. The inset shows the linear transformation of the data between the arrows, according to the integrated Michaelis-Menten equation adapted for enzymes with product inhibition, and the corresponding regression line. The reaction was recorded at 573 nm after reacting 30 nm dUTPase and 50 μM dUTP in the presence of 25 mM MgCl2, 2M BICINE, and 50 μM cresol red at pH 8.
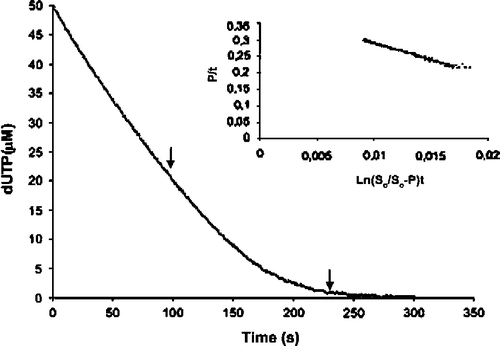
Figure 2. Inhibition by dUMP of dUTP hydrolysis. The Ki value for product (dUMP) inhibition was calculated from the Kmapp values obtained at different dUMP concentrations (5 to 100 μM). The Ki for dUMP was 11.4 μM and the real Km 0.66 μM.
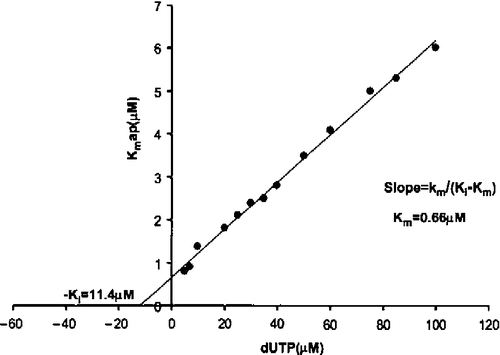
Figure 3. Inhibition by dUMP of dUDP hydrolysis. The Ki value for product (dUMP) inhibition was calculated with the Kmapp value obtained at different dUMP concentrations (5 to 100 μM). The Ki for dUMP was 50 μM and the real Km 4.74 μM.
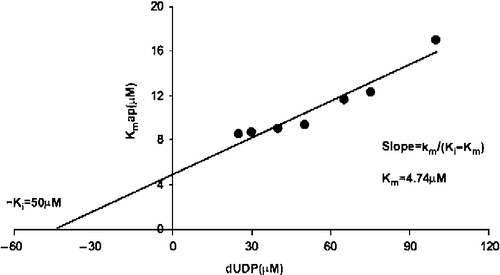
Table III. Inhibition constants Ki (μM) for compounds against Campylobacter jejuni, P. falciparum and human dUTPases.