Figures & data
Figure 1 . Representative scheme of the glutathione pathway and ABC-transporters family role in drug elimination and defence. Glutathione S-transferases (GSTs) conjugate GSH to drugs and drug metabolites facilitating the ATP-dependent elimination of drugs or drug-GS conjugates, involving ABC transporters (like ABCB1, ABCC1 and ABCG2). The drug/drug metabolites and intracellular GSH equilibrium is a major determinant of blood drug level.
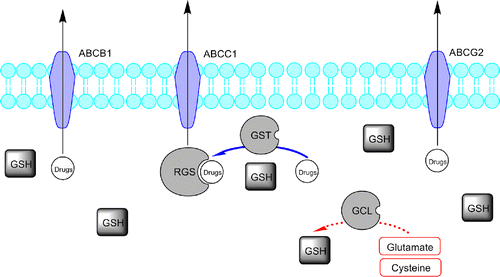
Figure 2 . Chemical structures of the green tea catechins and of the alkylating anti-cancer drugs docked. The oxygen numbering scheme and the ring nomenclature [Citation43] used throughout the text is indicated only for ( − )-EGCG.
![Figure 2 . Chemical structures of the green tea catechins and of the alkylating anti-cancer drugs docked. The oxygen numbering scheme and the ring nomenclature [Citation43] used throughout the text is indicated only for ( − )-EGCG.](/cms/asset/b4890c3e-f870-4cbb-aa1e-149bad4808f8/ienz_a_317895_f0002_b.gif)
Figure 3 . Left: binding into hGST P1-1 of ( − )-EGCG red, ( − )-GCG yellow, ( − )-EGC blue and ( − )-CG green. Right: binding into hGST P1-1of cyclophosphamide gray, ifosfamide light blue, melphalan pink and chlorambucil gold. The protein is represented as a light grey Connolly surface, while the ligands as stick models.
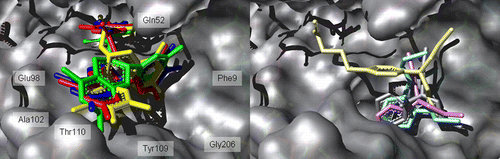
Table I. Heavy atoms (HA), molecular weight (MW) and QM/MM interaction energies (kcal/mol) for the eight studied compounds.
Figure 4 . Left: 3D structure of hGST P1-1 (solid ribbon) in complex with ( − )-EGCG (top) and ( − )-GCG (bottom). Residues lining the ligand position are represented as a white Connolly surface, while the ligands are represented by a green Connolly surface. Right: binding mode of ( − )-EGCG (top) and ( − )-GCG (bottom) within hGST P1-1. Ligand (CPK) and the residues involved in interactions (orange, labelled) are represented in stick while the hydrogen bonds are shown as green dotted lines.
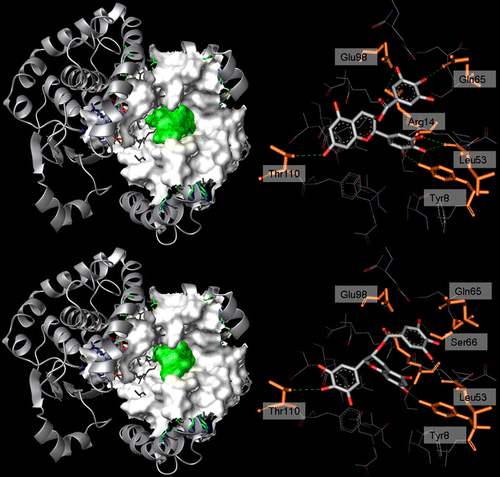
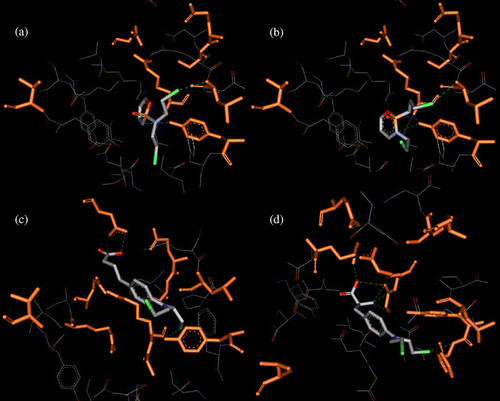
Figure 5 . Binding within hGST P1-1 of the considered drugs: cyclophosphamide (a), ifosfamide (b), chlorambucil (c) and melphalan (d). Ligand (CPK) and interacting key residues (orange) are represented as stick models, hydrogen bonds are shown as green dotted lines.