Figures & data
Figure 2. Total antioxidant activities of silymarin and standard antioxidant compounds such as BHA, BHT, α-tocopherol and trolox at the same concentration (30 μg/mL) assayed by ferric thiocyanate method (BHA: butylated hydroxyanisole, BHT: butylated hydroxytoluene).
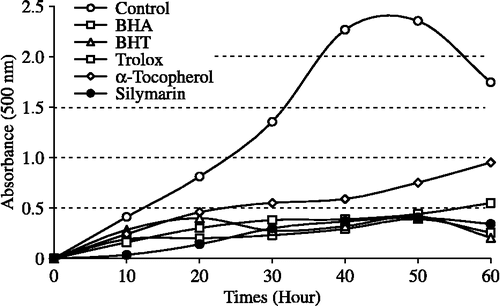
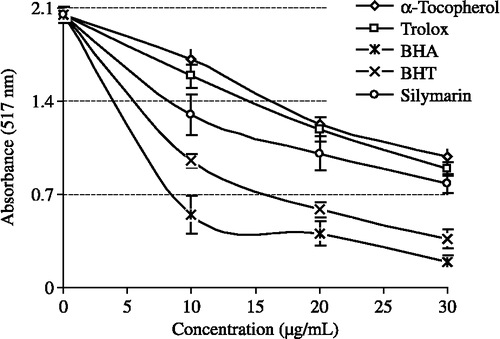
Figure 3. Total Fe3+ → Fe2+ reductive potential of different concentrations (10–30 μg/mL) of silymarin (r2:0.9177) and reference antioxidants; BHA, BHT, α-tocopherol and trolox (BHA: butylated hydroxyanisole, BHT: butylated hydroxytoluene).
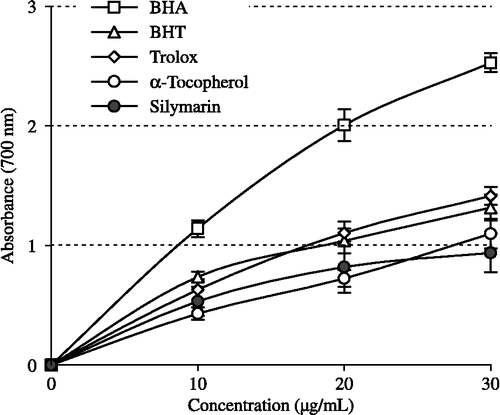
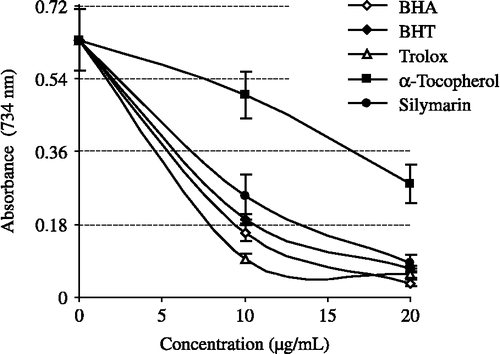
Figure 4. Cupric ions (Cu2+) reducing ability of different concentrations (10–30 μg/mL) of silymarin (r2:0.9768), BHA, BHT, α-tocopherol and trolox by Cuprac method (BHA: butylated hydroxyanisole, BHT: butylated hydroxytoluene).
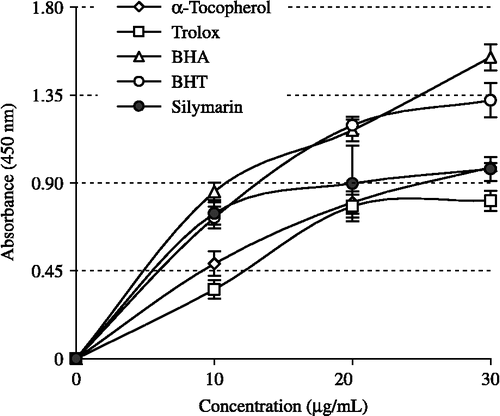
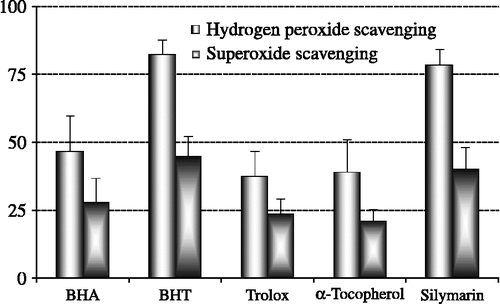
Figure 5. Comparison of hydrogen peroxide (H2O2) and superoxide anion radical (O2√ − ) scavenging activities of silymarin and standard antioxidant compounds such as BHA, BHT, (α-tocopherol and trolox at the concentration of 30 μg/mL (BHA: butylated hydroxyanisole, BHT: butylated hydroxytoluene).