Figures & data
Figure 1. The inhibition of FAS by (+)-catechin heated in sulfuric acid. (A): Time course of remaining FAS activity by inhibition of (+)-catechin treated with 1M sulfuric acid at 100°C. The concentration of (+)-catechin was 2.5 mg/mL. A 1.2-μl aliquot was taken to measure the remaining activity of FAS in a 2 mL assay mixture at the indicated times. (B): Time course of remaining FAS activity by inhibition of (+)-catechin treated with 1 M sulfuric acid at different temperatures. The concentration of (+)-catechin was 2.5 mg/mL. A 2-μl aliquot was taken to measure the remaining FAS activity in a 2 mL assay mixture at the indicated times. ▴: 100°C; ♦: 60°C; ★: 25°C. (C): Time course of remaining FAS activity by inhibition of (+)-catechin treated with different concentrations of sulfuric acid at 100°C. The concentration of (+)-catechin was 2.5 mg/mL. A 2-μl aliquot was taken to measure the remaining FAS activity in a 2 mL assay mixture at the indicated times. ▴: 1 M sulfuric acid; ♦: 0.5 M sulfuric acid; ★: 0.1 M sulfuric acid; •: water. The data was the mean of three experiments.
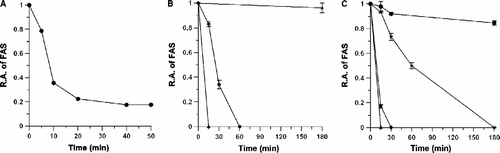
Table I. (+)-catechin treatment at 100°C with 1 M hydrochloric acid or organic acids.
Table II. IC50 values of different inhibitors of FAS in overall reaction (OA) and ketoacyl reduction (KR).
Figure 2. Double-reciprocal plots for inhibition of FAS by EG-2. (A): The overall reaction of FAS was measured. The concentrations of malonyl-CoA and NADPH were fixed at 9.25 μM and 33.4 μM, respectively. Acetyl-CoA was a variable substrate. The concentrations of EG-2 were 0 (♦); 0.16 μg/mL (▪); 0.33 μg/mL (•); 0.50 μg/mL (▴). (B): The β-ketoacyl reduction reaction was measured. The concentration of ethyl acetoacetate was fixed at 50 mM. NADPH was a variable substrate. The concentrations of EG-2 were 0 (♦); 0.50 μg/mL (▪); 1.00 μg/mL (•); 1.50 μg/mL (▴).
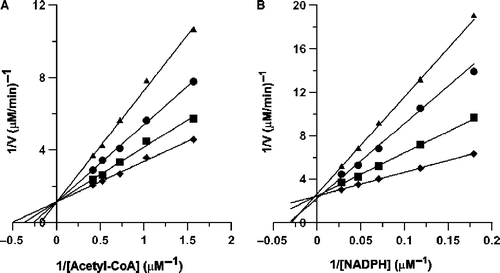
Figure 3. Impact of EGCG and EG-2 on MCF-7 cell viability. After been seeded in the 96-well plate, the breast cancer cells, MCF-7, were incubated with different concentrations of EGCG or EG-2 for 24 h. The percentage of viable cells was determined by MTT assay. Data are represented as means ± SD (n = 3). *Significantly different (p < 0.05) from control cells (no EGCG or EG-2) by Tukey test. **Significantly different (p < 0.01) from control cells (no EGCG or EG-2) by Tukey test.
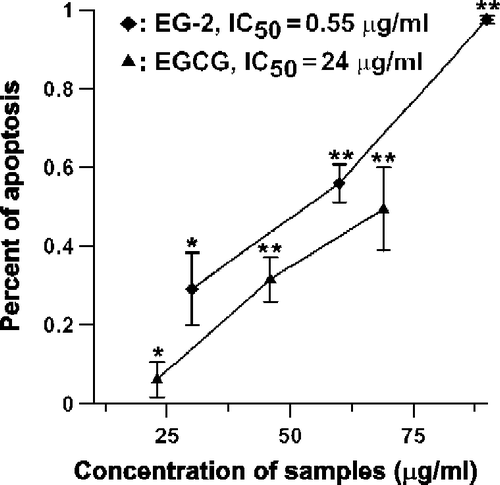
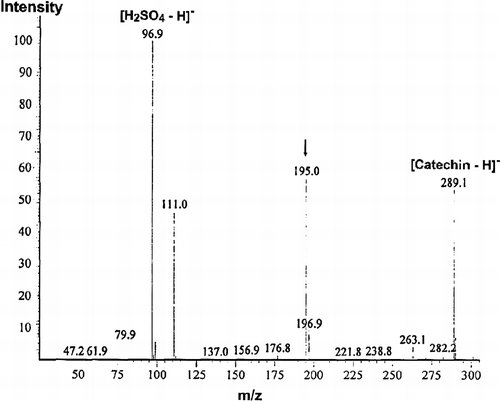
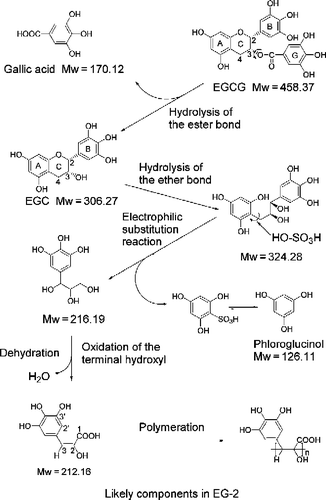
Figure 4. HSCCC chromatogram of EG-2 and MALDI-TOF mass spectrum of one of active fractions in EG-2. (A): Separation of active components from EG-2. Conditions: Two-solvent system: ethyl acetate-butanol-H2O (4:0.6:5, v/v/v); 0–6 h, in reverse-phase partition mode (mobile phase: aqueous lower phase); 6–9 h, in normal-phase partition mode (mobile phase: organic upper phase); flow rate: 2 mL/min; revolution speed: 850 rpm; sample concentration: 600 mg/30 mL. (B): The MALDI-TOF mass spectrum of an active fraction that was separated from Fraction 3 in EG-2.
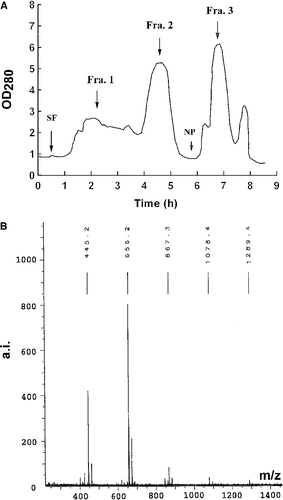
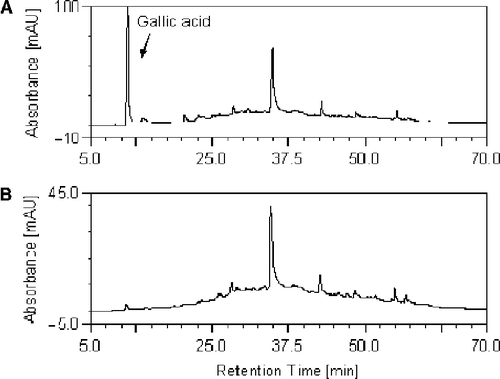
Figure 5. The HPLC chromatogram of E-2 and EG-2. The chromatogram of EG-2 and E-2 are shown in (A) and (B) respectively. The binary mobile phases consisted of 0.1% H3PO4 in water (Solvent A) and 0.1% H3PO4 in methanol (Solvent B). The system was run with a total gradient program, 5% Solvent B to 100% Solvent B in 60 minutes. The flow rate was 1 mL/min, detected at 254 nm.