Figures & data
Figure 2. (A) Disappearance of beauvericin in HLM at various initial concentrations. Beauvericin at concentrations of 40, 100, 400, and 1000 nM was incubated with 0.2 mg/mL HLM. Each point represents the logarithmic value of the remaining percentage of substrate to the initial concentration in HLM. (B) Plots of in vitro depletion rate constants versus substrate concentration for HLM-catalyzed beauvericin metabolism. Each point represents the depletion rate constants of corresponding substrate concentration. The line represents the curve predicted from Equation (1).
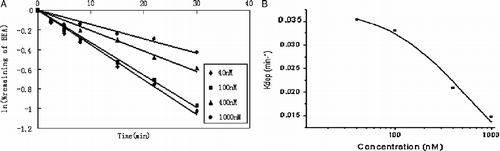
Table I. Inhibition effect of Beauvericin on cytochrome P450 isozyme-specific activities in HLM and RLM.
Figure 3. Inhibitory effects of beauvericin on CYP-catalyzed reactions in HLM (A) and RLM (B). Beauvericin were incubated under conditions described in Methods. The activity of each isoform was measured by isoform-specific substrate reaction probes at approximately their respective Km value. Each data point represents the mean of duplicate experiments.
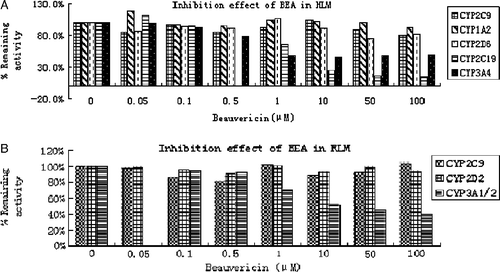
Figure 4. Inhibitory effect of beauvericin on CYP3A4/5 and 2C19 in HLM, and CYP3A1/2 in RLM for the value of IC50, using the software of Grafit, version 5.0. (A) Inhibition of CYP3A4/5 (with midazolam [5 μM] as substrate) by beauvericin (0 to 100 mM) in HLM; (B) Inhibition of CYP2C19 (with mephenytoin [55 μM] as substrate) by beauvericin (0 to 100 mM) in HLM; (C)) Inhibition of CYP3A1/2 (with midazolam [5 μM] as substrate) by beauvericin (0 to 100 mM) in RLM (see Materials and Methods for details). Data are averages for duplicate incubations.
![Figure 4. Inhibitory effect of beauvericin on CYP3A4/5 and 2C19 in HLM, and CYP3A1/2 in RLM for the value of IC50, using the software of Grafit, version 5.0. (A) Inhibition of CYP3A4/5 (with midazolam [5 μM] as substrate) by beauvericin (0 to 100 mM) in HLM; (B) Inhibition of CYP2C19 (with mephenytoin [55 μM] as substrate) by beauvericin (0 to 100 mM) in HLM; (C)) Inhibition of CYP3A1/2 (with midazolam [5 μM] as substrate) by beauvericin (0 to 100 mM) in RLM (see Materials and Methods for details). Data are averages for duplicate incubations.](/cms/asset/a0effdea-6863-4e38-b0ad-98e4842ab96b/ienz_a_336371_f0005_b.gif)
Figure 5. Lineweaver-Burk plots, Dixon plots, and secondary reciprocal plots of CYP3A4/5 catalyzed midazolam 1′-hydroxylation and CYP2C19-catalyzed mephenytoin 4′-hydroxylation by beauvericin (1–5 μM) in HLM. The symbols indicate mean of the transformed data for two independent experiments, and the lines were generated by linear regression analysis. Lineweaver-Burk plots of midazolam (A, 1–5 μM) and mephenytoin (D, 10–100 μM) in the presence of 0.5( × ), 1 (▴), 2 (▪), and 5 (♦) μM beauvericin. Dixon plots of midazolam (B) with 1 (♦), 2.5 (▪), and 5 (▴)μM and mephenytoin (E) with 10 ( × ), 20 (▴), 50 (▪), and 100 (♦) μM in the presence of beauvericin (0.5, 1, 2, and 5 μM). Secondary plots of the slopes taken from Lineweaver-Burk plots versus the beauvericin concentration for midazolam (C) and mephenytoin (F). Each data point represents the average of duplicate measurements.
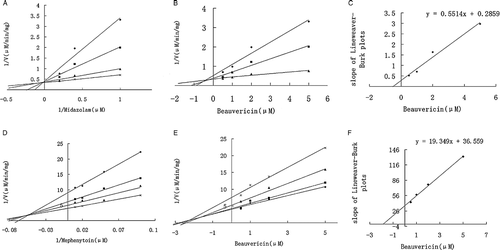
Figure 6. Typical chromatograms of beauvericin and phenytoin (internal standard, IS) in rat plasma. Plasma sample spiked with beauvericin (A) and IS (B); Blank plasma sample (C).
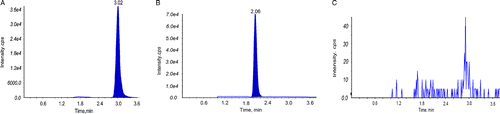
Figure 7. (A) Profiles of mean plasma concentration of beauvericin versus time after p.o. administration at the dose of 0.5, 1, 2 mg/kg. (B) Comparative profiles of mean plasma concentration of beauvericin after 0.5 mg/kg dose of beauvericin alone and co-administration with ketoconazole both at the dose of 0.5 mg/kg to rats. (C) Comparative profiles of mean plasma concentration of ketoconazole after 0.5 mg/kg dose of ketoconazole alone and co-administration with beauvericin both at the dose of 0.5 mg/kg to rats.
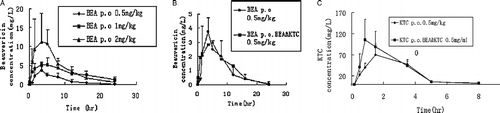
Table II-A. Pharmacokinetic parameters of beauvericin after oral administration to rats (n = 5; mean±S.D.).
Table II-B. Pharmacokinetic parameters of ketoconazole after oral administration to rats (n = 5; mean±S.D.).
![Figure 1. Full-scan product ion spectra of [M + H]+ and fragmentation pathways for beauvericin.](/cms/asset/d3bcf73c-0f76-46a4-a156-bef9692b2518/ienz_a_336371_f0002_b.gif)