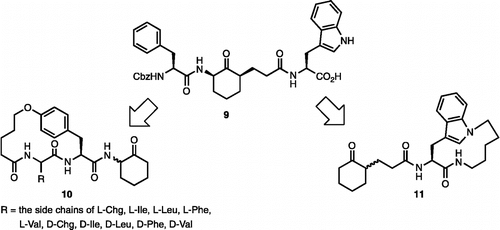
Figures & data
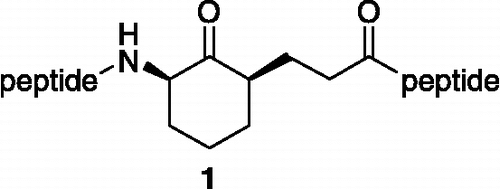
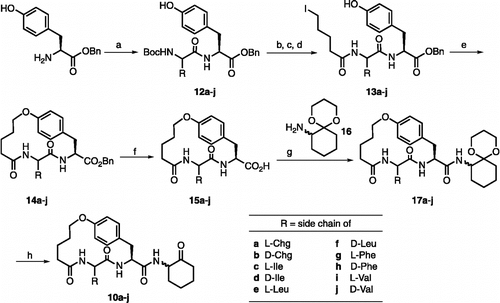
Scheme 1. Reagents and conditions: (a) Boc-aa-OH, HBTU, DIEA, rt, 2 h (86-99%); (b) TFA/CH2Cl2 (1:2), rt, 30 min; (c) 20% K2CO3, 5-bromopentanoyl chloride, rt, 8 min; (d) NaI, acetone, reflux, 2 h (88-100%); (e) K2CO3, rt, 10 h (50-75%); (f) H2, Pd(OH)2/C, rt, 4 h; (g) 16, HBTU, DIEA, rt, 24 h (60-80%); (h) TFA/H2O (1:2), rt, 12 h (50-65%). Compounds 13c, 13d and 13f were not isolated in pure form, but instead the crude materials were used directly in the next reaction. Compounds 17a-j and 10a-j are 1:1 mixtures of two diastereomers where the stereochemistry of the R substituent is defined, but the stereocenter on the cyclohexane ring is not.
Scheme 2. (a) LDA (2 equiv), 0°C, then 1-bromo-4-butene, rt, 30 h (78%); (b) 2 N NaOH:MeOH (1:1), reflux, 24 h (90%); (c) 1,3-propanediol, TMSCl, 0°C to rt, 48 h (95%); (d) NaIO4, KMnO4, NaHCO3, acetone/water (2:1), rt, 4 h (90%).
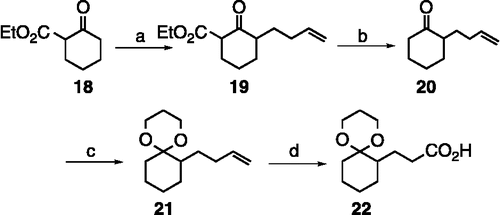
Scheme 3. (a) 5-amino-1-hexanol, HBTU, DIEA, rt, 2 h (95%); (b) TsCl, pyridine, rt, 2 h (97%); (c) NaH, rt, 2 d (30%); (d) TFA/CH2Cl2 (1:2), rt, 30 min; (e) 22, HBTU, DIEA, rt, 2 h (70% for two steps); (f) TFA/H2O (1:2), rt, 12 h (55%).
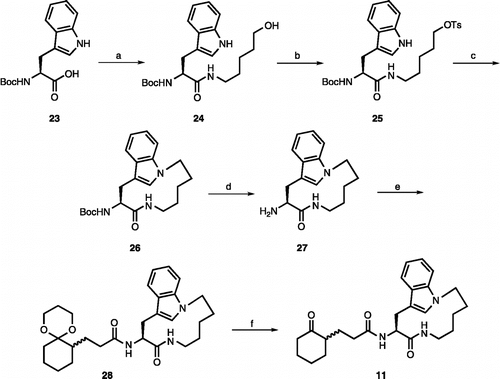
Figure 4. Assay results for inhibitors 10a-j (250 μM) against plasmin. The R group in each inhibitor is defined by the side chain of the amino acid shown on the x-axis of the plot. The data are an average of three independent measurements.
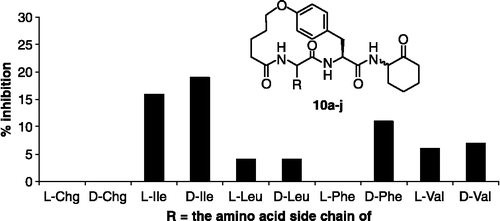
Table I. IC50 values of inhibitors 10c, 10d and 10h against plasmin.