Figures & data
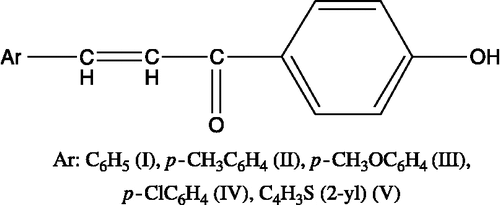
Table I. Cytotoxicity of the compounds against Jurkat cells, Log P and Hammett values of the substituents on the p- position of the phenyl ring.
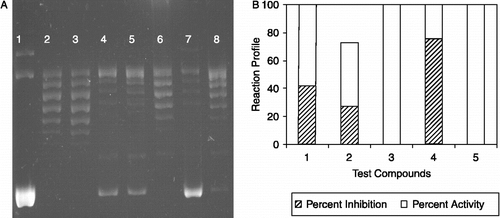
Figure 2. The effect of 4′-hydroxychalcone derivatives on mammalian DNA topoisomerase activity. A. A representative agarose gel photograph of supercoil relaxation with 1 unit of DNA topoisomerase I in the presence of varying concentrations of 4′-hydroxychalcone derivatives (see “Materials and Methods” for the details). Lane 1, plasmid substrate, pBR322 with no enzyme; lane 2, pBR322 with 1 u of DNA topoisomerase I; lane 3, same as lane 2 in the presence of DMSO, lanes 4 to 8, pBR322 with 1 u DNA topoisomerase I in the presence of 1 μg/μL of test cpds from I to V.B. Quantitative assessment of the inhibitions obtained with the compounds. DNA bands were quantified from gel photographs and plotted with the relationship between the binding of EtdBr and the amount of fluorescence given by sc and rlx DNA under UV light.