Figures & data
Figure 1. 17β-HSD1 catalyzes the reduction of estrone (E1) into estradiol (E2) with the cofactor NADH or NADPH.
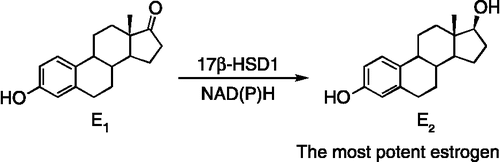
Figure 2. Chemical structure of the bisubstrate inhibitors of 17β-HSD1: EM-1745 (1) and its C17-ketone analogue 2. Illustrated only for 1 and 2, the stereogenic centers are the same for all other steroid derivatives reported in this paper.
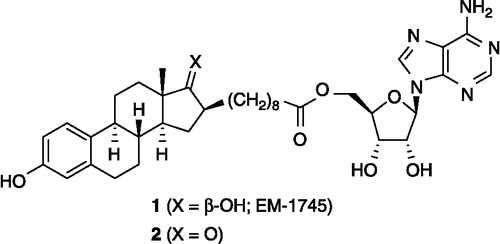
Table I. 13C NMR data for compounds 2, 7-11.
Scheme 1. A new procedure for preparing EM-1745 (1) and synthesizing the C17-ketone analogue of EM-1745 (2). Reagents, conditions and yields: (a) NaI, acetone, reflux, 16 h; (b) i. CuI, vinylMgBr, THF, − 40°C, 15 min; ii. HMPA, P(OEt)3, THF, − 40°C to rt, 5 h (75% for two steps); (c) TPAP, NMO, molecular sieves, DCM, rt, 90 min (51%); (d) 5, 2nd generation Grubbs' catalyst, DCM, reflux, 16 h (50%); (e) 10% Pd/C, H2, EtOAc, rt, 16 h; (f) NaClO2, NaH2PO4, THF, 2-methyl-2-butene, t-BuOH, rt, 30 min (75% for two steps); (g) 2′,3′-isopropylidene adenosine, PyBOP, HOBt, DIPEA, DMF, rt, 16 h (51%); (h) HClg, DCM, rt, 3 h (27%); (i) PPTS, DCM, MeOH, reflux, 5 h; (j) TBAF, THF, 0°C, 10 min (72% for two steps); (k) Jones' reagent, acetone, 0°C, 8 min (52%); (l) TFA/H2O, 9:1, THF, rt, 30 min (56%).
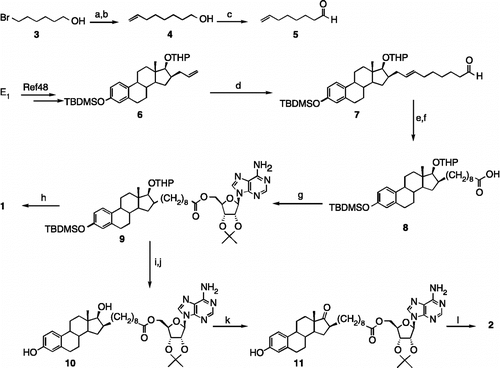
Figure 3. Inhibition of the transformation of [14C]-E1 into [14C]-E2 by EM-1745 (1) and its C17-ketone analogue 2 in homogenated HEK-293 cells everexpressing 17β-HSD1. See experimental section for more details. Data with the same symbol (* or **) are significantly different (P < 0.01).
![Figure 3. Inhibition of the transformation of [14C]-E1 into [14C]-E2 by EM-1745 (1) and its C17-ketone analogue 2 in homogenated HEK-293 cells everexpressing 17β-HSD1. See experimental section for more details. Data with the same symbol (* or **) are significantly different (P < 0.01).](/cms/asset/2fbfd86a-77b2-4ca6-9f80-dcee39d62236/ienz_a_340143_f0004_b.gif)
Table II. Inhibition of 17β-HSD1 by different kinds of inhibitors.