Figures & data
Table 1. Activities of 30 nM, 70 nM and 140 nM PFK-1 and AK.
Figure 1. Effects of LDH and ammonium sulfate on 30 nM PFK-1 activity. Solutions were prepared as given in Methods. After 1h dilution to 30 nM PFK-1, salt-free LDH (▪) was added at the final concentrations indicated and activities determined after 1h. Commercial LDH as a suspension in 3.2M ammonium sulfate (□) was added to 30 nM PFK-1 at the same final concentrations as salt-free LDH. Ammonium sulfate alone was added (▴) at the same final concentrations occurring in commercial LDH (shown in the secondary X axis).
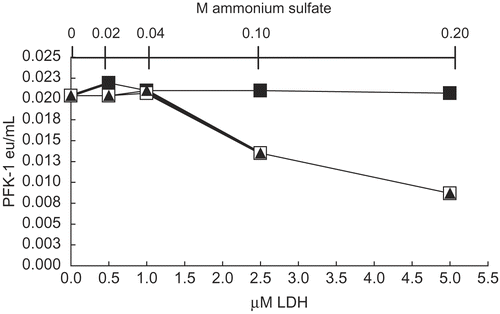
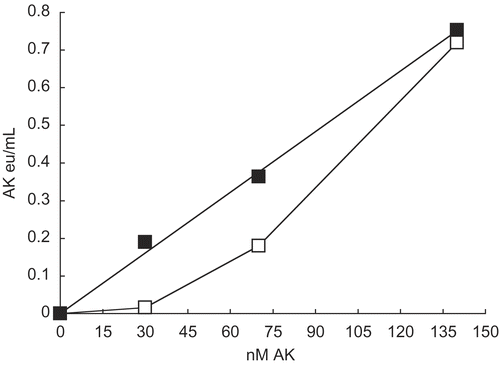
Figure 2. Effect of 0.2M ammonium sulfate on activity at 30 nM, 70 nM, and 140 nM AK. As given in Methods, AK was diluted with 0.02 M potassium phosphate, pH 8 to concentrations shown and allowed to stand 1 h as given in Methods. No additions (□) and 0.2 M ammonium sulfate (▪) were then made, incubated for 1 h and AK activities were then determined.
Figures 3 A-F. Relative activities of 30 nM (△), 70 nM (▴), and 140 nM (▪) PFK-1 and AK versus NH4+, Na+, and K+ion concentrations. The PFK-1 concentrations were derived from dilution of a 3 μM PFK-1stock solution and the preparation of samples is given in the Methods section. shows that NH4+, Na+, and K+ salts in decreasing order of effectiveness, resulted in recovery of some AK activity losses due to its low concentration. PFK-1 was treated in the same manner as AK and show that the presence of these salts results in losses of PFK-1 activities; K+, NH4+, and Na+ salts inhibited PFK-1 in decreasing order of effectiveness.
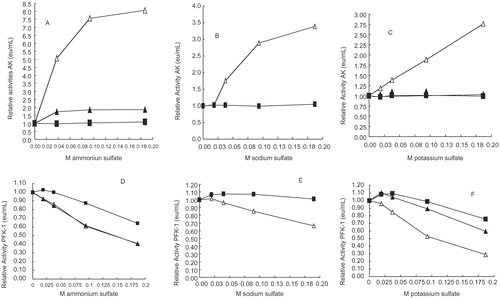
Figure 4. Comparison of effects on 30 nM AK activity by sulfates and chlorides of NH4+, K+, and Na+ and the effect of substrates. A 30 nM AK solution was prepared as described in Methods (see ) and solutions were made 0.2 molar with respect to each monovalent salts and 1 mmolar with respect to AMP or ATP.Mg when present. A 1 h incubation period followed and activities were then determined. The 30 nM AK control had an activity of 0.021 ± 0.002 eu/mL from 4 determinations.
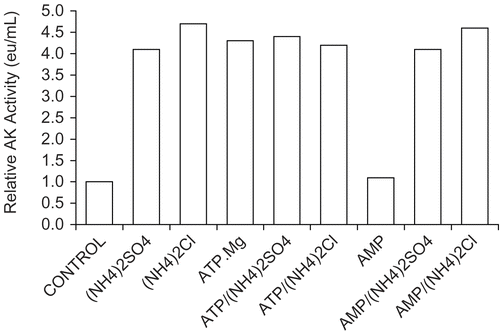
Table 2. The effect of 0.2 M sulfates of ammonium, potassium, and sodium on Km and Vm values of 30 nM and 140 mM AK.
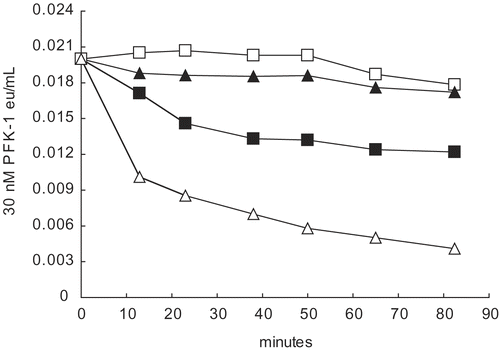
Figure 5. Effect of 0.2 M ammonium sulfate on 30 nM PFK-1 in the presence and absence of 5 μM aldolase. Dilutions to 30 nM PFK-1 (▪) were made with 0.1 M Tris-phosphate buffer, pH 8.0. The following were in 30 nM PFK-1 as final concentrations: 5 μM aldolase (□); 5 μM aldolase and 0.2 M ammonium sulfate (▴); and ammonium sulfate (△). The initial reading was at 13 minutes The 0-time value was based upon the estimated eu/mL values based on dilution shown in , Methods. Differences between aldolase alone (□) and aldolase and 0.2 M ammonium sulfate (▴) could not be shown to be significantly difference (p<0.01).