Figures & data
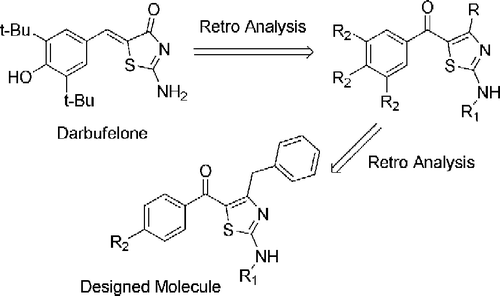
Scheme 1. Retroanalysis of Darbufelone (CI1004), a dual COX/LOX inhibitor. Pharmacophore generated from structure analysis of Darbufelone.
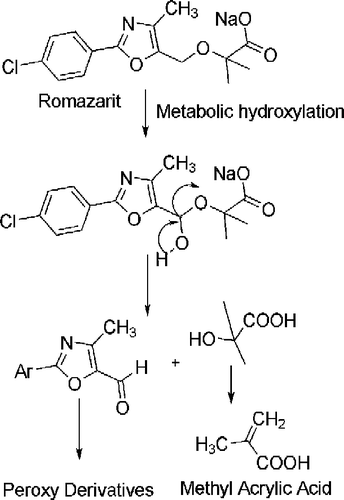
Scheme 2. Probable mechanism of metabolic toxicity exhibited by Romazarit. Peroxy type radicals or methyl acrylic acid moiety might be responsible for the bladder tumor formation.
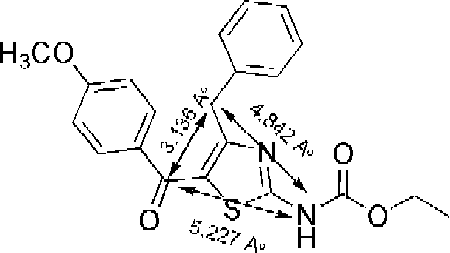
Scheme 4. Probable metabolic pathways of most potent compound RS31. The bioisosteric replacement of CH2 group at second position by NH obstructs the pathway that may generate peroxy type radicals or methyl acrylic acid moiety.
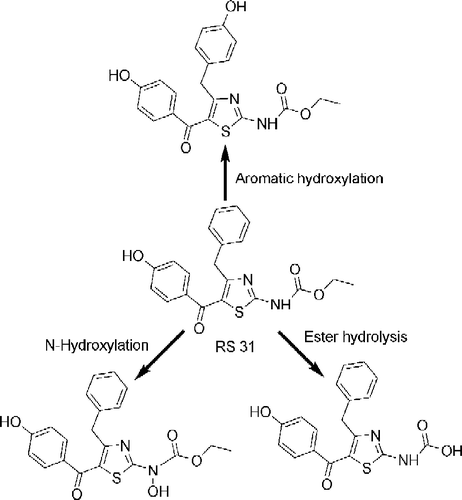
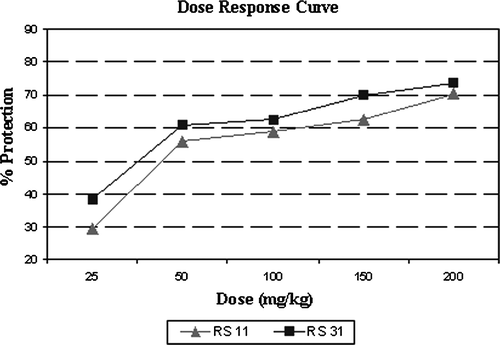
Table I. In vivo anti-inflammatory activity data of the 4-benzyl-1,3-thiazol derivatives.