Figures & data
Scheme 1. Reagents: (i) hydrazine hydrate, EtOH, reflux, 6 h; (ii) EtOH, reflux, 4 h; (iii) mercaptoacetic acid/2-mercaptopropionic acid, dry benzene, reflux, 6 h.
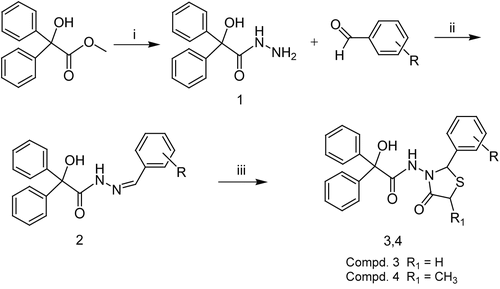
Table 1. The primary in vitro antimycobacterial activity of compounds 3a-r and 4a-r were determined against M. tuberculosis H37Rv using Microplate Alamar Blue Assay (MABA). The compounds were tested at a concentration of 6.25 µg/mL. The inhibition values of the compounds with more than 90% growth inhibition are indicated in boldface.
Table 2. In vitro tumor cell growth inhibition of 4p.