Figures & data
Figure 1. Optical microscopy images for SA-beads (upper panel) and PMCG microcapsules (lower panel) after encapsulation of M75 antibody (0 h) and after 7 d (168 h) of incubation in medium with pH 6.8 and 7.4. The scale bar is equal to 0.5 mm.
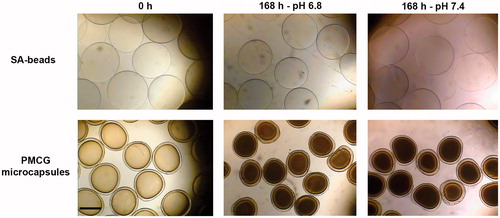
Figure 2. Compression curves expressed as compression force versus distance between probe and stand of Texture analyzer for SA-beads (upper panel) and PMCG microcapsules (lower panel) after encapsulation of M75 antibody (0 h) and after 7 d (168 h) of incubation in medium with pH 6.8 and 7.4.
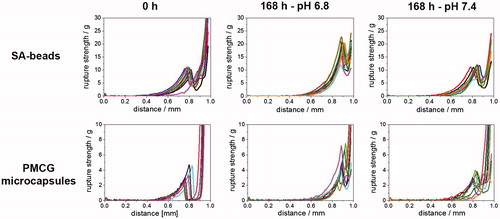
Figure 3. Quantification of encapsulated mouse immunoglobulins released into cultivation medium in quasi-static experiment. A: 0.5 ml aliquot of freshly prepared SA-beads and PMCG microcapsules were transferred into cultivation medium (either pH 6.8 or pH 7.4) and 0.5 ml of medium were taken out in time intervals of 1 h, 24 h, 48 h, 120 h, and 168 h. Withdrawn 0.5 ml medium was replaced by fresh one. B: Total release of M75 antibody in percent corresponding to theoretical content of antibody encapsulated in 0.5 ml of microspheres. IgG release was determined by commercially available ELISA kit for total mouse IgG detection and quantification.
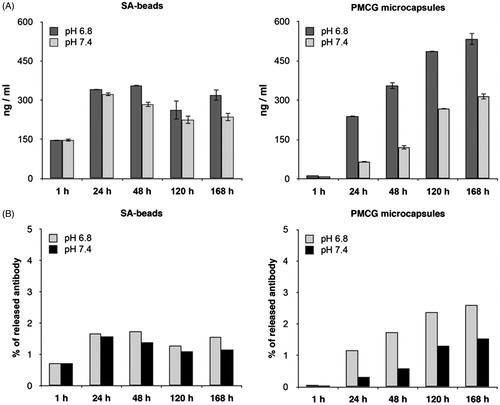
Figure 4. Immunoblotting of CA IX expression in different cell cultures. Protein lysates from CA IX-expressing (HeLa and MDCK CA IX) cells as well as negative cells (MDCK neo) were subjected to analysis using cultivation medium with the released M75 antibody. Characteristic twin band (54/58 kDa) appeared only in lines where proteins from CA IX-positive cells were loaded.
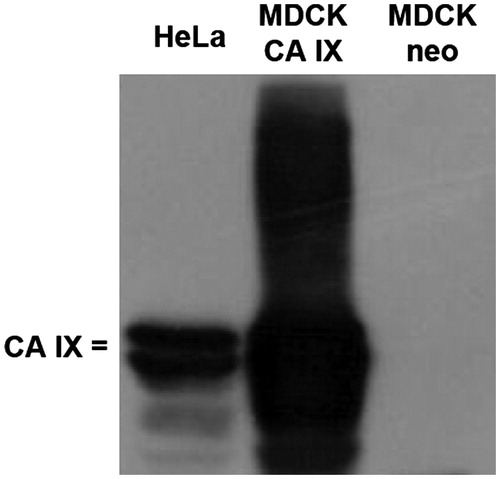
Figure 5. Immunofluorescence analysis of CA IX localization. MDCK CA IX as well as MDCK neo cells were stained with cultivation medium obtained after encapsulation of M75 antibody into both SA-beads as well as PMCG microcapsules followed by Alexa Fluor 488-conjugated secondary antibody. The positive CA IX-specific immunoreaction (green color) was localized at the cell membrane of the MDCK CA IX cells. Empty-vector transfected MDCK cells (MDCK neo) were negative. Cell nuclei were stained using DAPI (blue color). The scale bar is equal to 20 μm.
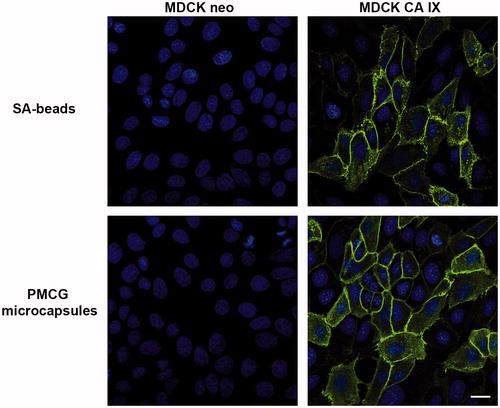
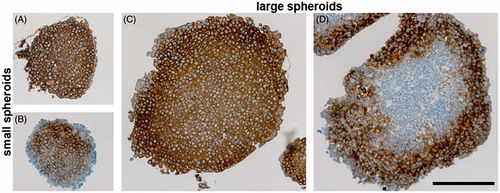
Figure 6. Immunohistochemical analysis of CA IX expression in either small (A and B) or large (C and D) spheroids from SiHa cells. A and C: Staining pattern of CA IX in spheroids cultivated without encapsulated antibody (positive control). Standard immunohistochemical staining of CA IX was performed with both primary (hybridoma medium diluted 1:100) as well as secondary antibody. CA IX-specific staining (brown color) is localized across the spheroid. B and D: Uptake of the encapsulated M75 antibody into SiHa spheroids that was determined by incubation of paraffin sections only in secondary anti-mouse antibody. All sections were counterstained with Mayer’s hematoxylin (blue nuclei). The scale bar is equal to 200 μm.