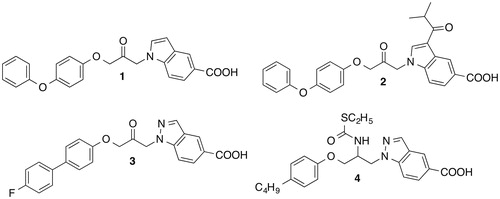
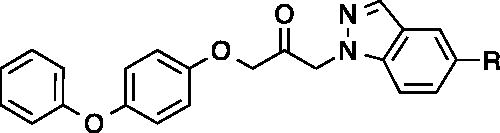
Figures & data
Scheme 1. Reagents and conditions: (a) 4-Phenoxyphenol, 4-(dimethylamino)pyridine, 120 °C, 45 min; (b) Dess-Martin periodinane, CH2Cl2, room temp., 2.5 h; (c) 10% aqueous KOH, ethanol, room temp., 14 h.
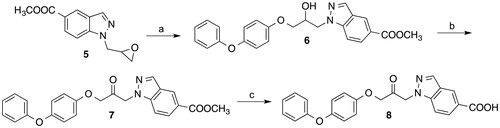
Scheme 2. Reagents and conditions: (a) Cs2CO3, DMF, room temp., 23 h; (b) Dess-Martin periodinane, CH2Cl2, room temp., 3 h.
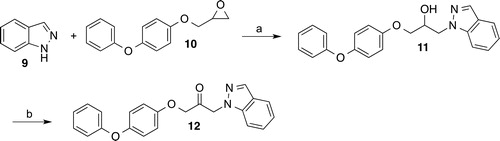
Scheme 3. Reagents and conditions: (a) 15: Acetic anhydride, THF, triethylamine, room temp., 1.5 h; 18: isobutyryl chloride, pyridine, 0 °C, 2 h; 22: di-tert-butyl dicarbonate, methanol, Amberlyst® 15, room temp., 2 h; 26: 2-methoxyacetyl chloride, pyridine, 0 °C, 2 h; 30: 2-benzyloxyacetyl chloride, diisopropyl(ethyl)amine, DMF, 0 °C to room temp., 4 h; 34: methyl chloroformate, pyridine, 0 °C, 2 h; (b) 15: epichlorohydrin, Cs2CO3, acetonitrile, reflux, 3 h; 19, 23, 27, 31, 35: epichlorohydrin, Cs2CO3, DMF, room temp., 16–24 h; (c) 4-phenoxyphenol, 16, 32: Cs2CO3, tetrabutylammonium bromide, acetonitrile, reflux, 4–7 h; 20: 4-(dimethylamino)pyridine, DABCO, DMF, 120 °C, 1 h; 24: DABCO, DMF, 120 °C, 6 h; 28: DABCO, 4-(dimethylamino)pyridine, DMF, 120 °C, 4 h, 36: 4-(dimethylamino)-pyridine, 120 °C, 2 h; (d) 17, 21, 29, 37: acetic anhydride, DMSO, room temp., 12–16 h, 25: Dess-Martin periodinane, CH2Cl2, room temp., 4.5 h followed by trifluoroacetic acid, CH2Cl2, room temp., 18 h, 33: Dess-Martin periodinane, CH2Cl2, room temp., 3 h; (e) benzoyl chloride, ethyl acetate, pyridine, room temp., 2 h; (f) H2, Pd/C, THF, room temp., 4 d.
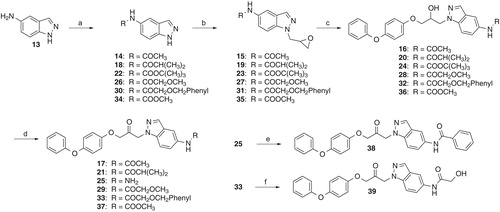
Scheme 4. Reagents and conditions: (a) 2-[(4-Phenoxyphenoxy)methyl]oxirane, Cs2CO3, DMF, room temp., 72 h; (b) tert-butyldimethylsilyl chloride, imidazole, THF, room temp., 2 d; (c) H2, Pd/C, THF, 5 h; (d) 44: phenyl chloroformate, THF, room temp., 1 h; 47: phenyl isocyanate, THF, room temp., 30 min; (e) CuCl2 dihydrate, acetone, H2O, reflux, 40 h (45) or 8 h (48); (f) Dess-Martin periodinane, CH2Cl2, room temp., 1.5 h (48) or 4 h (49).
![Scheme 4. Reagents and conditions: (a) 2-[(4-Phenoxyphenoxy)methyl]oxirane, Cs2CO3, DMF, room temp., 72 h; (b) tert-butyldimethylsilyl chloride, imidazole, THF, room temp., 2 d; (c) H2, Pd/C, THF, 5 h; (d) 44: phenyl chloroformate, THF, room temp., 1 h; 47: phenyl isocyanate, THF, room temp., 30 min; (e) CuCl2 dihydrate, acetone, H2O, reflux, 40 h (45) or 8 h (48); (f) Dess-Martin periodinane, CH2Cl2, room temp., 1.5 h (48) or 4 h (49).](/cms/asset/d6b5305a-f134-41bb-bd21-6c2ba6f3ec55/ienz_a_1178246_sch0004_b.jpg)
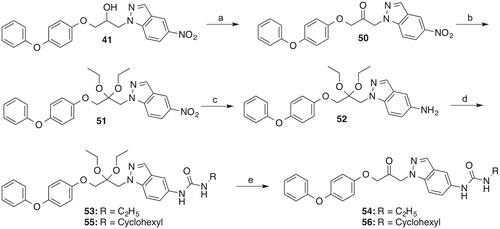
Scheme 5. Reagents and conditions: (a) Dess-Martin periodinane, CH2Cl2, room temp., 4 h; (b) triethyl orthoformate, H2SO4 conc., ethanol, reflux, 12 h; (c) H2, Pd/C, THF, 5.5 h; (d) 53: ethyl isocyanate, THF, room temp., 1 d, 55: cyclohexyl isocyanate, THF, room temp., 6 d; (e) 54: H2O, acetone, water, H2SO4 conc., room temp., 2 d, 56: ethanol, acetone, THF, water, H2SO4 conc., room temp., 4 d.
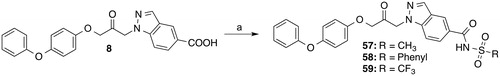
Scheme 6. Reagents and conditions: (a) Methanesulfonamide (57), benzenesulfonamide (58) or trifluoromethanesulfonamide (59), 4-(dimethylamino)pyridine, CH2Cl2, EDC, room temp., 20–42 h.