Figures & data
Figure 1. Chemical structures of traditional NSAIDs; aspirin (1), indomethacin (2) and ibuprofen (3), selective COX-2 inhibitors; celecoxib (4), rofecoxib (5), and valdecoxib (6) and reported pyrazolo[3,4-d]pyrimidine derivatives (7–9) with anti-inflammatory activity.
![Figure 1. Chemical structures of traditional NSAIDs; aspirin (1), indomethacin (2) and ibuprofen (3), selective COX-2 inhibitors; celecoxib (4), rofecoxib (5), and valdecoxib (6) and reported pyrazolo[3,4-d]pyrimidine derivatives (7–9) with anti-inflammatory activity.](/cms/asset/741de061-1a8b-4eb5-96ac-b67d73540ca8/ienz_a_1186018_f0001_b.jpg)
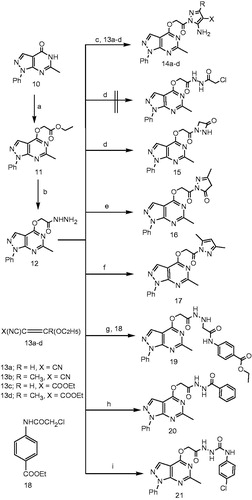
Scheme 1. Reagents and conditions: (a) ethyl chloroacetate, acetone, K2CO3, reflux, 6 h; (b) hydrazine hydrate, ethanol, reflux, 5 h; (c) CR(OC2H5)=C(CN)X, ethanol, 10 h; (d) chloroacetyl chloride, DMF, K2CO3, RT, overnight; (e) ethyl acetoacetate, ethanol, reflux, 12 h; (f) acetylacetone, CH3COOH, reflux, 6 h; (g) 4-COOC2H5-C6H4-NHCOCH2Cl, ethanol, reflux, 8 h; (h) benzoyl chloride, ethanol, reflux, 6 h; (i) 4-chlorophenylisocyanate, dioxane, reflux, 4 h.