Figures & data
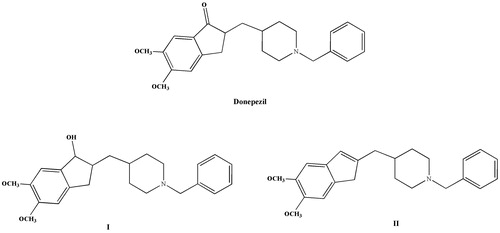
Figure 1. Structures of some AChE inhibitors: donepezil and indanol- and indene-based derivatives (I and II) reported as AChE inhibitors.
Scheme 1. Reagents and conditions: (a) oxalyl chloride, CH2Cl2, room temperature, 12 h; (b) AlCl3, CH2Cl2, 0 °C, ice bath; (c) Dimethyl carbonate, NaH, 90 °C, reflux; (d) appropriate aniline, dioxane, microwave; (e) NaBH4, THF, MeOH, 2 h; (f) MeOH, PTSA, reflux, 25 min. *R1, R2, R3 substituent groups at the o-, m- and p- positions: H, CH3, C2H5, OCH3, OC2H5, F, Cl and Br.
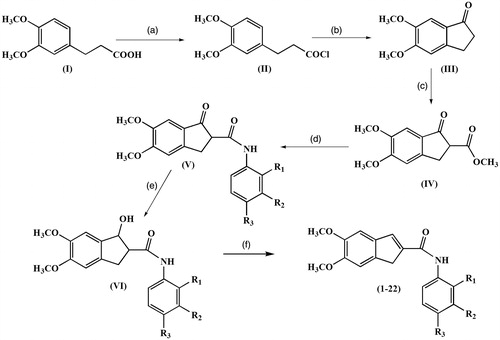
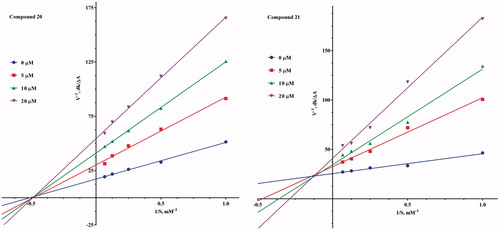
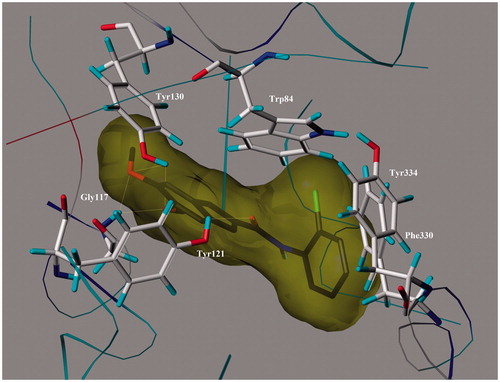
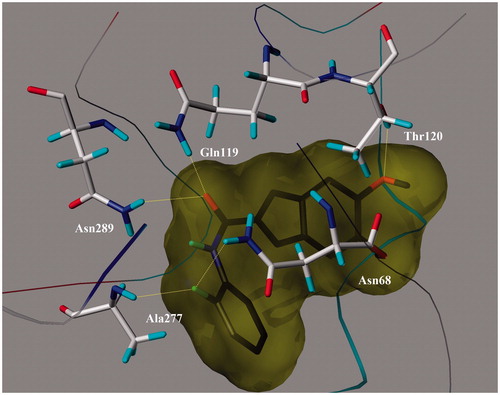
Table 1. Anticholinesterase activity, inhibition of self-induced Aβ1–42 aggregation of the compounds (1–22).
Figure 3. Inhibition of self-induced Aβ1–42 aggregation by the test compounds and reference curcumin at concentration 25 μM.
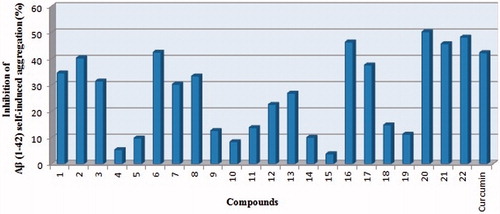
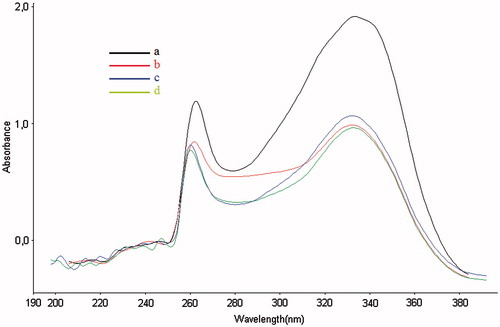
Figure 4. (a) UV absorption spectra of compound 20 (100 μM in DMSO) alone, (b) UV absorption spectra of the mixture compound 20 (100 μM) and CuSO4 (100 μM), (c) UV absorption spectra of the mixture compound 20 (100 μM) and FeSO4 (100 μM) and (d) UV absorption spectra of the mixture compound 20 (100 μM) and ZnSO4 (100 μM).